I posted a poll asking the following question: Check out the lab results below. This patient is most likely to have:
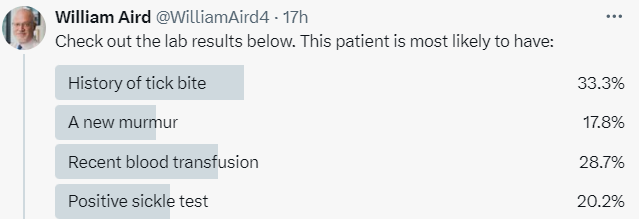
The most popular answer was history of a tick bite.
The correct answer was a new heart murmur, which was the least favorite choice!
Let’s work through this together:
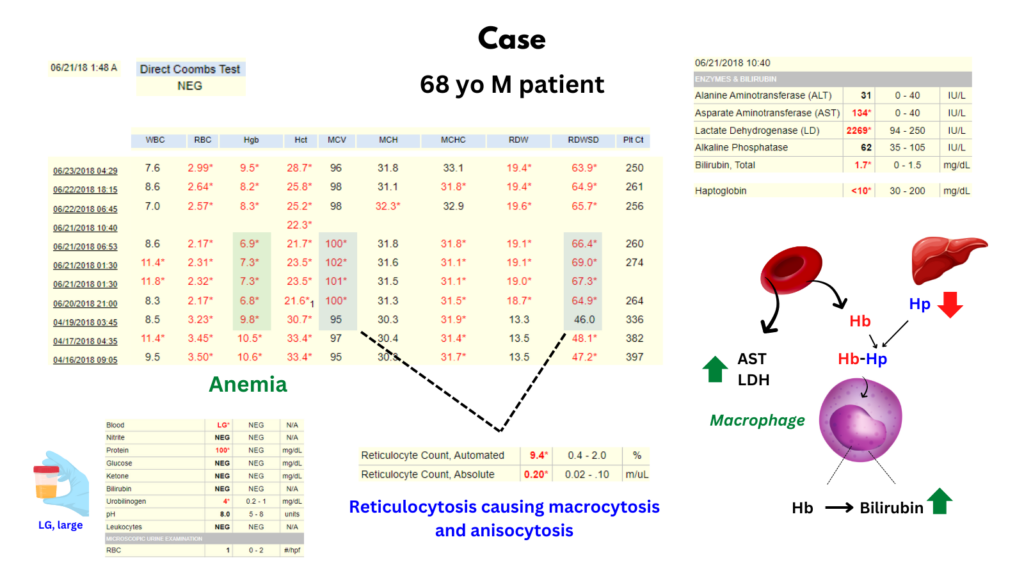
- The patient has hemolytic anemia, as evidenced by:
- Decreased Hb
- Increased reticulocyte count (absolute reticulocyte count > 0.12 x 1012/L)
- Hemolytic markers
- Blood
- Elevated:
- AST
- LDH
- Bilirubin
- Decreased haptoglobin
- Elevated:
- Hemoglobinuria (dipstick positive for blood, microscopy negative for red cells)
- Blood
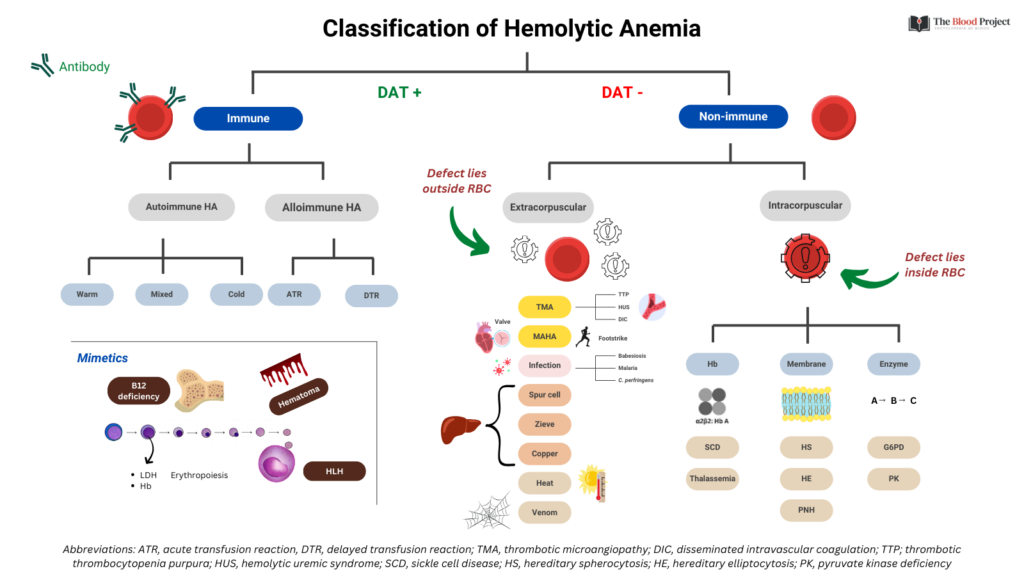
- Hemolytic anemia may be:
- Immune – positive for direct antiglobulin test (DAT, Coombs test)
- Non-immune – negative for DAT
- The DAT was negative, thus the patient’s hemolytic anemia is of the non-immune variety
- Non-immune hemolytic anemia may be:
- Intracorpuscular
- Extracorpuscular
- Intracorpuscular non-immune hemolytic anemia:
- Includes:
- Membranopathy, including:
- Hereditary spherocytosis
- Hereditary elliptocytosis
- PNH
- Hemoglobinopathy, including:
- Sickle cell disease
- Thalassemia
- Enzymopathy, including:
- G6PD deficiency
- PK deficiency
- Membranopathy, including:
- All cause of intracorpuscular hemolytic anemia are congenital except PNH and rare cases of acquired G6PD deficiency.
- This patient has acute on chronic anemia, unlikely to be explained by congenital membranopathy, hemoglobinopathy or enzymopathy; we cannot rule out sickle cell disease with pain crisis, but the CBC would normally show leukocytosis and/or a change in platelet count and would not typically reveal hemoglobinuria.
- Acquired membranopathy (PNH) remains a possibility. However, none of the questions in the poll relate to this diagnosis (e.g., does the patient have early morning hematuria).
- Includes:
- Extravascular non-immune hemolytic anemia:
- Possibilities include:
- Thrombotic microangiopathy:
- Disseminated intravascular coagulation
- Thrombotic thrombocytopenia purpura
- Hemolytic uremia syndrome
- Liver disease:
- Zieve syndrome
- Spur cell anemia
- Wilson disease
- Infection:
- Malaria
- Babesiosis – associated with:
- Hemolytic anemia
- Thrombocytopenia – not present in this case
- Transaminitis (both ALT and AST) – only AST elevated in this case (from hemolysis)
- Mechanical intravascular hemolysis:
- Valves – often associated with development of a new murmur!
- Cardia assist devices
- Marathon running
- Bongo drumming
- Thrombotic microangiopathy:
- Possibilities include:
To review the answers offered in the poll:
- History of tick bite (incorrect) – Babesiosis would typically cause thrombocytopenia and transaminitis in addition to hemolytic anemia. Anaplasmosis does not cause hemolytic anemia.
- A new murmur (correct) – Associated with paravalvular leak from a defective prosthetic valve and intravascular hemolysis.
- Recent blood transfusion (incorrect) – An acute or chronic transfusion reaction would be associated with a positive direct antiglobulin test.
- Positive sickle test (incorrect) – Occurs with sickle cell trait and sickle cell disease. Formally possible, but the CBC typically shows more abnormalities, and urine hemoglobinuria is rare.
Summary of case
68-year-old man s/p MV repair with Gore-Tex neochordae and mitral valve annuloplasty on 4/5/18, now presenting with non-immune intravascular hemolytic anemia with ++schistocytes on peripheral smear. TTE 6/21/18 showed well seated mitral annuloplasty ring with normal gradients and mild to moderate valvular mitral regurgitation. TEE 5/22/18 showed well-seated mitral annuloplasty ring. Moderate anterior leaflet mitral valve prolapse with at least moderate, high-velocity mitral regurgitation. Normal left ventricular systolic function. Compared with the prior TEE (images reviewed) of 04/05/2018 , a mitral annuloplasty ring was now seen with at least moderate mitral regurgitation. The database was diagnostic of vale hemolysis. Medical treatment included supportive care with red blood cell transfusions, iron, folate and hydration (the latter to minimize the risks of AKI from hemoglobinuria. He ultimately underwent redo mitral valve repair with resolution of hemolysis.
See infographic on valve hemolysis.
Introduction
- Mechanical intravascular hemolysis occurs when red blood cells (RBCs) are fragmented inside blood vessels by mechanical injury, typically in the setting of prosthetic valves and mechanical assist devices.1
Definitions
- Mechanical intravascular hemolysis (aka cardiac prosthesis-related hemolytic anemia [CPHA]) defined by:2
- New hemolysis diagnosed in patients with:
- Cardiac prostheses (valve hemolysis)
- Mechanical assist devices
- Absence of other causes of hemolysis.
- New hemolysis diagnosed in patients with:
- Hemolytic anemia defined by:3
- Otherwise unexplained anemia.
- Signs of accelerated red blood cells (RBCs) production in the bone marrow (e.g., high reticulocyte count).
- Signs of RBCs destruction (e.g. elevated unconjugated bilirubin, lactate dehydrogenase [LDH], low haptoglobin).
- Sub-clinical hemolysis is used to describe patients who meet the latter two criteria but do not have anemia owing to adequate bone marrow compensation.
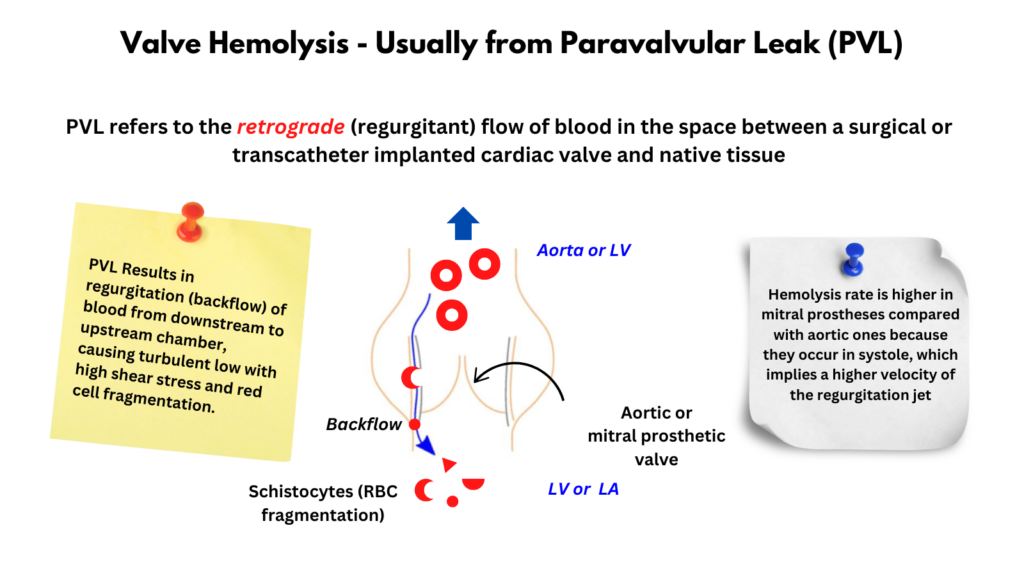
- Paravalvular leak (PVL):45
- PVL refers to the retrograde (regurgitant) flow of blood in the space between a surgical or transcatheter implanted cardiac valve and native tissue.6
- Mechanical vs. bioprosthetic implants:7
- Mechanical heart valves are fashioned from metal or carbon compounds.
- Bioprosthetic valves are contrived from either animal or human pericardial or valvular tissue:
- Shorter life‐span
- More structural deterioration
History
- In 1961, Sayed and co-workers reported severe hemolysis in a man who underwent surgical repair using a Teflon patch to close an atrial septal defect and postulated that hemolysis was produced by a jet stream of blood striking a non-endothelized portion of the Teflon patch.8
- In 1964, Reed and Dunn reported a case of fatal traumatic hemolysis following the insertion of a Starr-Edwards aortic ball valve prosthesis.9
- In 1966, Cooley summarized the literature (15 cases) regarding hemolysis following aortic valve replacement; he wrote:10
- “Intravascular hemolytic anemia and bizarre red blood cell fragmentation has been an infrequent but spectacular complication of open heart surgery.”
- “This syndrome has occurred in association with defective intracardiac prosthetic implants, both interatrial patch grafts and valve prostheses.”
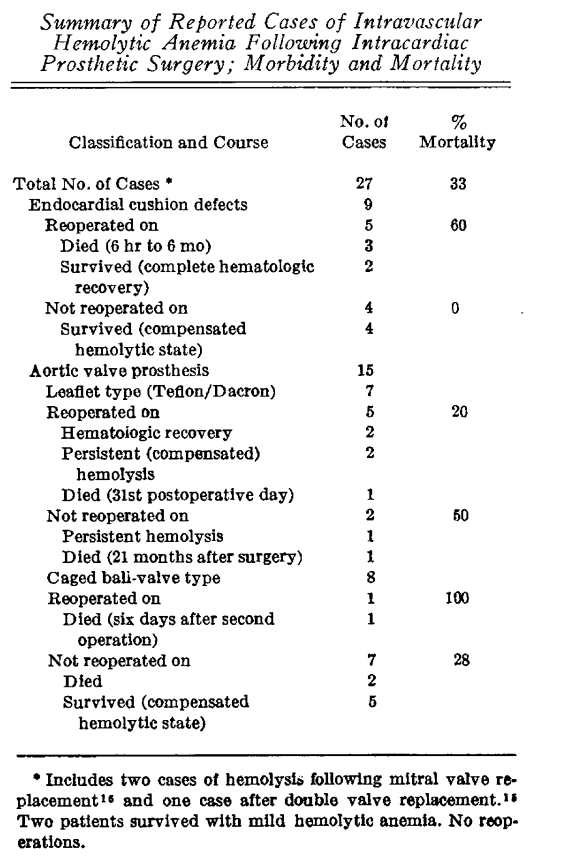
- In 1968, Taguchi et al reported a fatal case of severe hemolytic anemia following replacement of both the aortic and mitral valves with Starr-Edwards prostheses.11
- In 1968, Kastor et al reported on the incidence of PVL and hemolysis in patients receiving Starr-Edwards prosthetic valves:12
- Aortic valves:
- 27 of 203 patients (13 per cent) developed paravalvular aortic leaks.
- 11 patients had hemolytic anemia.
- Mitral valves:
- 10 patients of 108 (9%) developed paravalvular aortic leaks.
- Hemolytic anemia developed in 2 patients.
- Aortic valves:
- In 1975, Kloster reported the incidence of hemolysis in patients with prosthetic valves to be between 5 and 15%.13
Pathogenesis
- Valve- and cardiac device-related intravascular hemolysis:14
- Caused by:
- Increased shear stress:
- Arising from:
- Paravalvular leak (by far the most common scenario).
- Tight native aortic valve stenosis within a high velocity jet (very rare).
- Resulting in red blood cell shape change, deformation and membrane stretching, ultimately leading to hemolysis.
- Arising from:
- Direct contact between RBCs and prosthetic material in the internal surfaces of the heart.
- Increased shear stress:
- Hemolysis rate is higher in mitral prostheses compared with aortic ones because they occur in systole, which implies a higher velocity of the regurgitation jet.15
- Caused by:
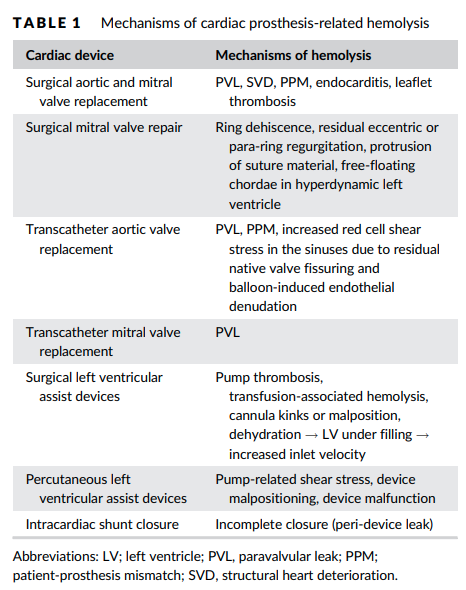
- Mechanisms of hemolysis according to the procedure:16
- Surgical aortic and mitral valve:
- Paravalvular leak (PVL)
- Patient‐prosthesis mismatch (PPM)
- Structural heart deterioration
- Surgical mitral valve repair:
- Ring dehiscence
- Residual eccentric or para‐ring regurgitation
- Protrusion of suture material
- Free‐floating chordae in hyperdynamic left ventricle
- Transcatheter aortic valve replacement:
- PVL
- PPM
- Increased red cell shear stress in the sinuses due to residual native valve fissuring
- Balloon‐induced endothelial denudation
- Transcatheter mitral valve replacement:
- PVL
- Surgical left ventricular assist devices:
- Pump thrombosis
- Transfusion‐associated hemolysis
- Cannula kinks or malposition
- Dehydration → LV under filling → increased inlet velocity
- Percutaneous left ventricular assist devices:
- Pump‐related shear stress
- Device malpositioning
- Device malfunction
- Surgical aortic and mitral valve:
- Paravalvular leak (PVL):1718
- PVL is the mot common cause of hemolysis after surgical valve replacement.
- Characterized by a gap between the prosthetic valve and the native annular tissue.19
- Results in regurgitation (backflow) of blood from downstream to upstream chamber, causing turbulent low with high shear stress and red cell fragmentation.
- Causes of PVL include:20
- Tissue friability
- Suture dehiscence due to heavy annular calcifications
- Endocarditis
- Chronic steroids
- Suboptimal surgical technique21
- Anemia may be accentuated in cases of:22
- Ineffective erythropoiesis, where the RBC destruction rate is not compensated by the production of new RBCs.
- High cardiac output (including from preexisting anemia), where RBC fragmentation is increased.
- Increased RBC fragility, for example:23
- Iron deficiency
- Hereditary spherocytosis
- Hereditary elliptocytosis
- Bleeding from acquired von Willebrand deficiency, especially in context of left ventricular outflow tract (LVOT).
Epidemiology
| Procedure | PVL | Subclinical hemolysis | Hemolytic anemia |
|---|---|---|---|
| Native valves | NA | NR | Stenotic aortic valves > stenotic mitral valves |
| Surgical valves | 5–10% (aortic) 7-17% (mitral) | 18-51% (mechanical) 5-10% (tissue) | <1% |
| Transcatheter TAVR | 50–85% (overall) 1.6-2% (mod-severe) | 15%-37% | Rare |
| Transcatheter TMVI | 10% | NR | NR |
| Transcatheter MV repair | NR | 8% | 1-3.3% |
- Valve replacement:[efn_note]PMID 31039274[/efn_note]
- Surgical valves:24
- Paravalvular leak (PVL):
- 2–10% in the aortic position; risk factors include:
- Valve replacement in a supra-annular position
- Use of continuous suture
- 7–17% in the mitral position; risk factors include:25
- Older age
- Endocarditis
- Renal dysfunction
- Atrial size
- The vast majority of these are diagnosed in the first year after surgery.26
- 2–10% in the aortic position; risk factors include:
- Hemolysis:
- Mechanical valves:
- Hemolytic anemia:
- Occurred in up to 15% of surgical valves in the 1960s to 1970s.
- Incidence decreased to <1% with modern valve designs.
- Subclinical hemolysis:
- Frequently observed in patients with contemporary mechanical prostheses.
- Incidence ranges from 18-51%.
- Hemolytic anemia:
- Bioprosthetic:
- Hemolytic anemia rare.
- Subclinical hemolysis in 5-10%.
- Mechanical valves:
- Paravalvular leak (PVL):
- Percutaneous valves:
- Transcatheter aortic valve replace (TAVR):
- Paravalvular leak (PVL):
- Estimated incidence of 50–85% (most are mild).
- Moderate to severe PVL occurs in approximately 2.5% of patients.
- Hemolysis:
- Hemolytic anemia rare.
- Subclinical hemolysis in 15%-37%.
- Paravalvular leak (PVL):
- Transcatheter mitral valve replacement (TMVR):
- Little data (see study below).
- Transcatheter aortic valve replace (TAVR):
- Surgical valves:24
- Valve repair:
Clinical Presentation
- Clinical presentation of paravalvular leak:
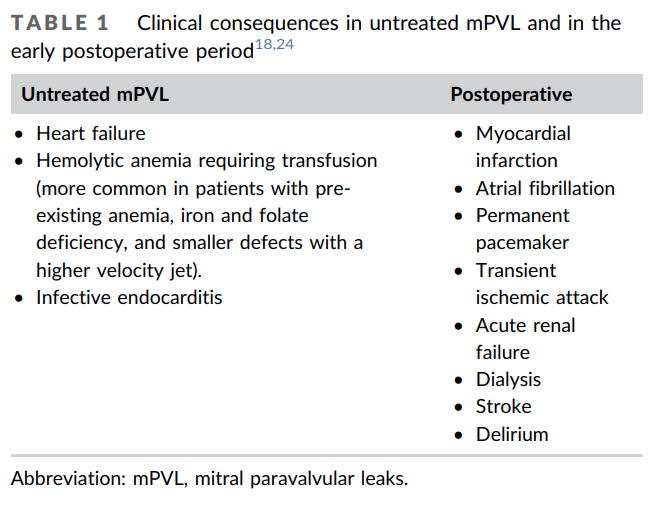
- Although most PVLs are small and remain asymptomatic and have a benign clinical course an estimated 1–5% of PVLs can progress and lead to more serious clinical consequences warranting repair, including:29303132
- Heart failure symptoms:
- Typically associated with large PVLs.
- Account for about 90% clinical presentation.
- Majority of those with congestive heart failure have a mean NYHA functional class of ≥3.
- Hemolytic anemia:
- More common in smaller defects with high-velocity jets.
- It has been estimated that between 33 and 75% of patients with symptomatic PVL will have clinically significant hemolysis.33
- Accounts for about one-third of presentations.34
- It is also possible to observe both hemolysis and heart failure symptoms due to PVL in the same patient (up to 50% of cases).35
- May present with:
- Asymptomatic laboratory findings of chronic intravascular hemolysis
- Insidious presentation with new onset fatigue
- Severe anemia36
- Jaundice
- Dark-colored urine
- Renal failure
- Congestive heart failure
- Infective endocarditis
- Heart failure symptoms:
- The majority of PVLs (74%) develop during the first year after surgery.37
- Although most PVLs are small and remain asymptomatic and have a benign clinical course an estimated 1–5% of PVLs can progress and lead to more serious clinical consequences warranting repair, including:29303132
Diagnosis
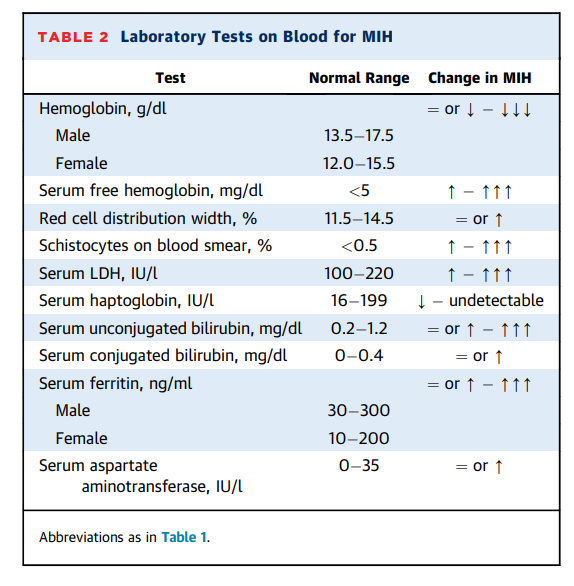
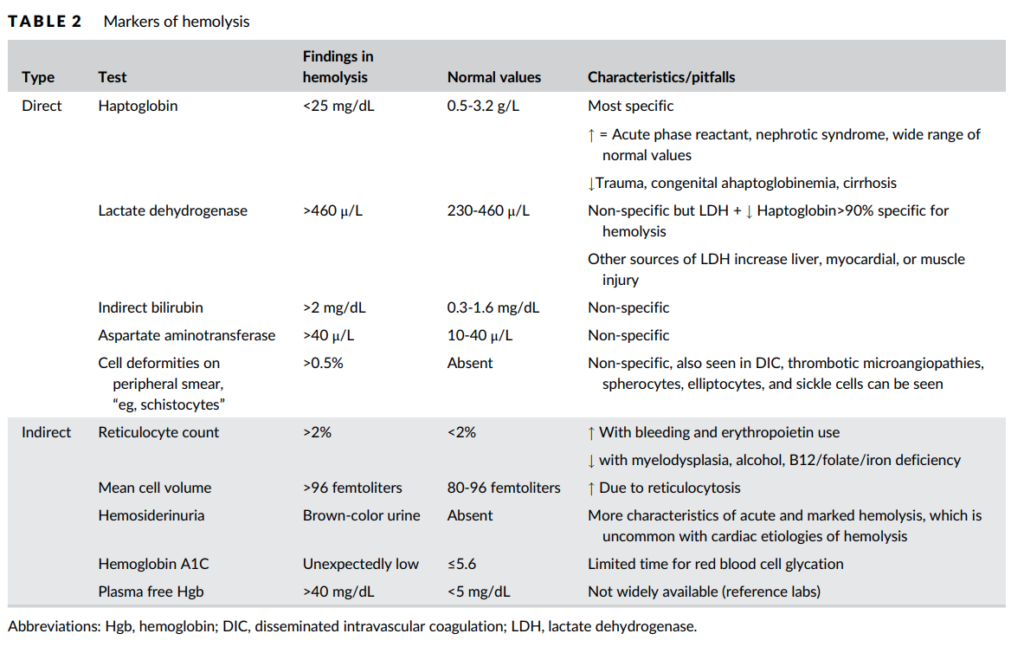
- Diagnosis of hemolytic anemia:
- Blood:
- Complete blood count (CBC):
- Elevated reticulocyte count
- Positive hemolytic indices:
- Elevated:
- LDH
- Indirect bilirubin
- AST
- Plasma free hemoglobin
- Decreased or absent haptoglobin
- Elevated:
- Peripheral smear showing schistocytes from mechanical fragmentation of RBCs
- Direct Coombs negative
- Urine:
- Dipstick positive for blood
- Microscopy negative for red blood cells
- Positive hemosiderin
- Blood:
- Diagnosis of paravalvular leak:40
- Transesophageal echocardiography
- Cardiac CT
- Cardiac MRI
Treatment
- Medical therapy:
- Hemolysis:41
- Folic acid42
- Iron supplements as needed.43
- Transfusion as needed, though no agreed upon threshold to transfuse.
- Erythropoietin:
- Has been shown to eliminate the need for transfusion in selected patients with prosthetic valve-related hemolysis:
- Recombinant human erythropoietin use in hemolytic anemia due to prosthetic heart valves: a promising treatment:
- Two patients with valve-induced hemolysis treated with erythropoietin.
- Patient #1:
- Starr-Edwards aortic valve replacement 24 y ago, MVR 4 y ago
- Developed valve hemolysis.
- Erythropoietin level was 68 mu/ml.
- Started on recombinant erythropoietin 10,000 units subcutaneously three x/ week.
- Hemoglobin started rising; subsequently switched to erythropoietin 40,000 units subcutaneously weekly.
- At 30-month follow-up he was maintaining Hb at 12.1g/dl.
- Patient #2:
- Mitral valve replacement 16 years ago and a subsequent replacement again approximately 10 years ago.
- Developed hemolysis.
- The patient’s erythropoietin level was only 25 mu/ml
- Started on erythropoietin at a dose of 40,000 units subcutaneously once every week.
- Hemoglobin increased to 12.1 g/dl and was maintained for 12 months despite persistent evidence of microangiopathic hemolytic anemia.
- Conclusions: “Although it might be too premature to recommend, looking into the possibility of use of recombinant human erythropoietin for patients with severe mechanical hemolysis may be warranted, especially in the presence of an inadequate erythropoietin level. This therapy is particularly useful inpatients in whom repeat surgery for valvular replacement contraindicated or to either minimize or avoid packed red blood cell transfusions.”
- Beta-blockers:
- Oral beta-blockers decrease in red blood cell shear stress in patients with PVL‐related hemolysis reducing blood pressure and heart rate.
- Have led to significant improvement in hemolytic anemia in several retrospective series.
- The effect of propranolol on hemolysis in patients with an aortic prosthetic valve:
- Introduction: “It was postulated that propranolol, by slowing the heart rate and decreasing the rate of ejection, might decrease turbulence and thereby decrease hemolysis. We present data on the use of propranolol in five patients with severe hemolysis.”
- 4 of 5 demonstrated an increase in red cell survival times with propranolol therapy; 3 of these showed a reduced RBC survival after propranolol withdrawal.
- The mean red cell half life for the five patients was 7.4 days at baseline, 9.8 days during propranolol therapy, and 8.6 days after propranolol withdrawal.
- Conclusions: “Propranolol was given to five patients with severe hemolytic anemia from aortic prostheses. Red cell survival, lactic dehydrogenase, serum hemoglobin, and 24 hour urine iron values were used to evaluate the severity of hemolysis with and without propranolol treatment. Three patients had a clear decrease in the level of hemolysis with propranolol therapy. One patient developed congestive failure after 6 months on propranolol. The decrease in hemolysis is most likely related to a slower heart rate.”
- Propranolol for intractable hemolysis after open heart operation:
- Patient had undergone open mitral commissurotomy for mitral stenosis.
- Seven years later exertional dyspnea developed, and cardiac catheterization revealed moderate mitral stenosis, mild mitral regurgitation, and severe tricuspid regurgitation.
- At operation, a severely deformed mitral valve was replaced with a St. Jude Medical prosthesis.
- On the 20th postoperative day, her urine was found to be black. A weak pansystolic blowing murmur was heard at the left sternal border. The hemoglobin value was 73 g/L, the maximum serum lactate dehydrogenase level was 7,525 IU, and the plasma free hemoglobin level was 879 mg/L. There was moderate hepatic and renal dysfunction. Peripheral blood examination for hemolysis excluded intrinsic erythrocyte abnormalities.
- Mechanical hemolysis due to a paramitral prosthetic valve leak was highly suspected, and 30 mg of propranolol (0.8 mg/kg) was prescribed on the 131st postoperative day.
- Six days after propranolol administration was started, her urine became clear, the lactate dehydrogenase level declined to 1,480 IU, and the plasma hemoglobin was 52 mg/L.
- When propranolol was discontinued, hemolysis recurred.
- Pentoxifylline:
- Pentoxifylline improves blood viscosity and red cell deformability.
- The use of pentoxifylline may subside mild hemolysis in patients with LVAD or mechanical valves.
- The effect of pentoxifylline on haemolysis in patients with double cardiac prosthetic valves:
- 40 patients who underwent double valve (mitral and aortic) replacement.
- The patients were randomly assigned to two groups as control (n = 20) and PTX group (n = 20).
- PTX was given in a daily oral dose of 1200 mg (3 times 400 mg) for 120 days.
- Laboratory tests for evidence of haemolysis namely, hemoglobin (Hb), hematocrit (Hct), plasma total bilirubin, indirect bilirubin and haptoglobin levels, corrected reticulocyte percent and serum lactic dehydrogenase activity (SLDH) were performed before and after the PTX treatment.
- PTX treatment caused significant increases in Hb, HTC, and haptoglobin levels (P < 0.05, P < 0.05 and P < 0.01, respectively). Additionally, there were significant decreases in SLDH, total and indirect bilirubin levels, and corrected reticulocyte percent in patients receiving PTX as compared with their respective control values (P < 0.01, for all).
- PTX treatment caused a significant improvement, to different extents, in signs of haemolysis in 60% of the patients. On the other hand, the response rate was 5% in the placebo-treated control group (P < 0.05).
- Conclusions: “These findings suggest that PTX may be an effective agent in the management of haemolysis in patients with prosthetic heart valves.”
- Guideline recommendations:
- 2022 ESC/EACTS Guidelines for the management of valvular heart disease:
- “Medical therapy (including iron supplementation, beta-blockers, and erythropoietin) is indicated in patients with severe haemolytic anaemia when contraindications to surgical or transcatheter closure are present.”
- 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease:
- “Medical therapy for prosthetic valve regurgitation is appropriate in asymptomatic patients or when the cause of regurgitation is valve thrombosis or prosthetic valve endocarditis, although further intervention may ultimately be needed in many of these patients. Some patients tolerate asymptomatic prosthetic valve regurgitation for many years, similar to patients with native valve regurgitation. However, there may be patients who develop rapid progression of the severity of bioprosthetic valve regurgitation because of leaflet degeneration. In patients with hemolytic anemia attributable to paravalvular regurgitation, medical management with folic acid and iron supplementation or periodic transfusion may be possible when the anemia is not severe, with intervention reserved for patients with symptomatic intractable anemia.”
- 2022 ESC/EACTS Guidelines for the management of valvular heart disease:
- Congestive heart failure:
- Diuretic therapy for volume overload
- Afterload reduction in patients with hypertension
- Endocarditis:
- Appropriate antibiotic therapy for endocarditis
- Hemolysis:41
- Surgical and transcatheter repair:
- Surgical reoperation is the gold standard therapy for symptomatic PVLs.
- More effective method in treating severe hemolysis than percutaneous repair44
- May carry moderate or high operative risk.
- Guideline recommendations:
- 2022 ESC/EACTS Guidelines for the management of valvular heart disease:
- Reoperation is recommended if the paravalvular leak is related to endocarditis or causes haemolysis requiring repeated blood transfusions or leading to severe symptoms. Transcatheter closure of a paravalvular leak is feasible, but experience is limited and there is presently no conclusive evidence to show consistent efficacy. Transcatheter closure of paravalvular leaks should be considered for anatomically suitable paravalvular leaks in candidates selected by the Heart Team.
- Reoperation is recommended if a paravalvular leak is related to endocarditis or causes haemolysis requiring repeated blood transfusions or leading to severe heart failure symptoms.
- Transcatheter closure should be considered for suitable paravalvular leaks with clinically significant regurgitation and/or haemolysis in patients at high or prohibitive surgical risk.
- Decision on transcatheter or surgical closure of clinically significant paravalvular leaks should be considered based on patient risk status, leak morphology, and local expertise
- Decision on transcatheter or surgical closure of clinically significant paravalvular leaks should be considered based on patient risk status, leak morphology, and local expertise.
- 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease:
- In patients with intractable hemolysis or HF attributable to prosthetic transvalvular or paravalvular leak, surgery is recommended unless surgical risk is high or prohibitive.
- In asymptomatic patients with severe prosthetic regurgitation and low operative risk, surgery is reasonable.
- In patients with prosthetic paravalvular regurgitation with the following: 1) either intractable hemolysis or NYHA class III or IV symptoms and 2) who are at high or prohibitive surgical risk and 3) have anatomic features suitable for catheter-based therapy, percutaneous repair of paravalvular leak is reasonable when performed at a Comprehensive Valve Center.
- For patients with severe HF symptoms caused by bioprosthetic valve regurgitation who are at high to prohibitive surgical risk, a transcatheter ViV procedure is reasonable when performed at a Comprehensive Valve Center
- 2022 ESC/EACTS Guidelines for the management of valvular heart disease:
Primary data
- Retrospective study of intravascular hemolysis after transcatheter aortic valve implantation self-expandable prosthesis:
- 94 consecutive patients treated with supra-annular self-expandable TAVI prosthesis.
- Incidence of haemolysis at 1-year follow-up varied between 9% and 28%, based on different hemolysis definitions.
- Haemolysis was mild in all cases.
- Presence of moderate/severe paravalvular aortic regurgitation was associated with hemolysis.
- The presence of hemolysis did not have any impact on patient prognosis after 6 years.
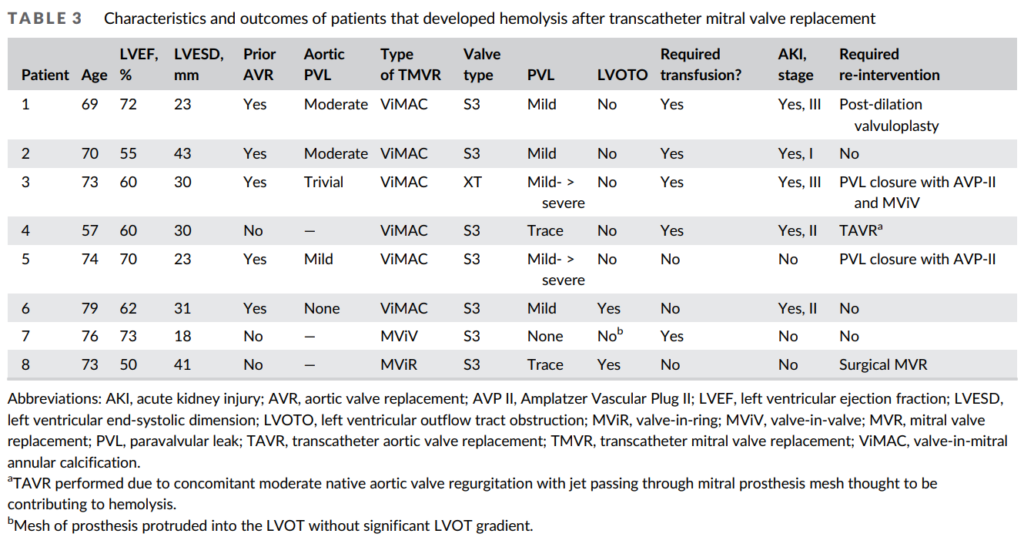
- Retrospective, single-center, observational study of patients undergoing transcatheter mitral valve replacement (TMVR):
- Patients underwent TMVR for:
- Degenerated mitral bioprostheses
- Failed annuloplasty rings
- Native valve disease
- All patients:
- Had symptomatic severe mitral valve disease.
- Were deemed to be high risk surgical candidates.
- The primary outcome was new hemolytic anemia within 6-months of TMVR:
- Hb decrease ≥2 g/dl from baseline without alternative explanation of anemia.
- Biochemical evidence of hemolysis with:
- Elevated lactate dehydrogenase
- Low haptoglobin
- 101 patients:
- 69 with mitral valve-in-valve (MViV)
- 14 with valve-in-ring (MViR)
- 18 with valve-in-mitral annular calcification (ViMAC)
- The majority of patients had both significant stenosis and regurgitation.
- ViMAC patients had an increased frequency of mild or greater paravalvular leak (PVL) (ViMAC, 72.2%; MViR, 14.3%; MViV, 13.0%; p < .001).
- Hemolysis:
- Occurred in a total of 8 patients:
- ViMAC, 6/18 (33.3%)
- MViR, 1/14 (7.1%)
- MViV, 1/69 (1.5%)
- Lab findings included:
- Changes in the blood:
- Mean peak decrease in hemoglobin from baseline was 4.8 ± 1.4 g/dl.
- Median lactate dehydrogenase was 1,130 U/L
- Median total bilirubin was 1.5 mg/dl
- All had an undetectable haptoglobin ( <14 mg/dl)
- Changes in the urine:
- Gross hemoglobinuria developed in four patients with ViMAC.
- Acute kidney injury occurred in five patients with hemolysis (all with ViMAC)
- Changes in the blood:
- All had clinical or biochemical evidence of hemolysis manifest within 1 week of TMVR.
- RBC transfusion was given in five patients with hemolysis (ViMAC, 4 and MViV, 1). P
- Occurred in a total of 8 patients:
- Authors conclusions:
- Hemolysis was uncommon after MViV or MViR but common after ViMAC and likely related to an increased frequency of PVL.
- Hemolysis was often clinically severe after ViMAC with the majority of cases associated with acute kidney injury and need for red blood cell transfusion.
- All patients with hemolysis after TMVR were identified early post-TMVR with symptoms or biochemical evidence presenting within 1 week post-procedure.
- Hemolysis was self-limited in both patients with MViV or MViR but persisted in ViMAC patients unless successful transcatheter re-intervention was performed.
- Patients underwent TMVR for:
- Review of hemolytic anemia following mitral valve repair:
- Review of 70 cases of hemolysis after mitral valve repair.
- Mitral regurgitation, as detected by transthoracic echocardiography, was severe in most patients, while only 8.57% of patients experienced mild regurgitation.
- The mechanism by which hemolysis occurred varied:
- Collision in 24 patients (28.57%)
- Acceleration (14 patients [16%])
- Fragmentation (16 patients [19%])
- Free jet (16 patients [19%])
- Slow deceleration (five patients [5.95%])
- Discussion” “Hemolysis is due either to the failure of the repair resulting in postoperative valvular leakage or to collision of the regurgitant blood with one of the structures of the valvular apparatus. The regurgitant blood can impact a residual chordae tendineae, a dehisced ring or an undehisced but rigid ring. The regurgitant jet itself perpetuates hemolysis by delaying endothelialization of the ring, causing further fragmentation and collision. The severity of hemolysis did not appear to correlate well with the severity of regurgitation, but correlated better with the degree of shear stress created during regurgitation of the jet.”
- Prospective study on the effect of percutaneous paravalvular leak closure on hemolysis:
- 168 patients who underwent percutaneous mitral (n= 130) or aortic (n= 38) PVL closure.
- mean time from surgical valve replacement to percutaneous PVL closure was 4.4+/-6.6 year.
- Follow-up hemolytic parameters were obtained within 7–30 days and 6 months.
- 70 patients (42%) had a study defined successful outcome, including:
- Increased hemoglobin
- Decreased LDH
- 48 (59%) patients had clinically successful hemolysis outcome.
- Eight patients (5%) experienced worsening of hemolysis following PVL closure.
