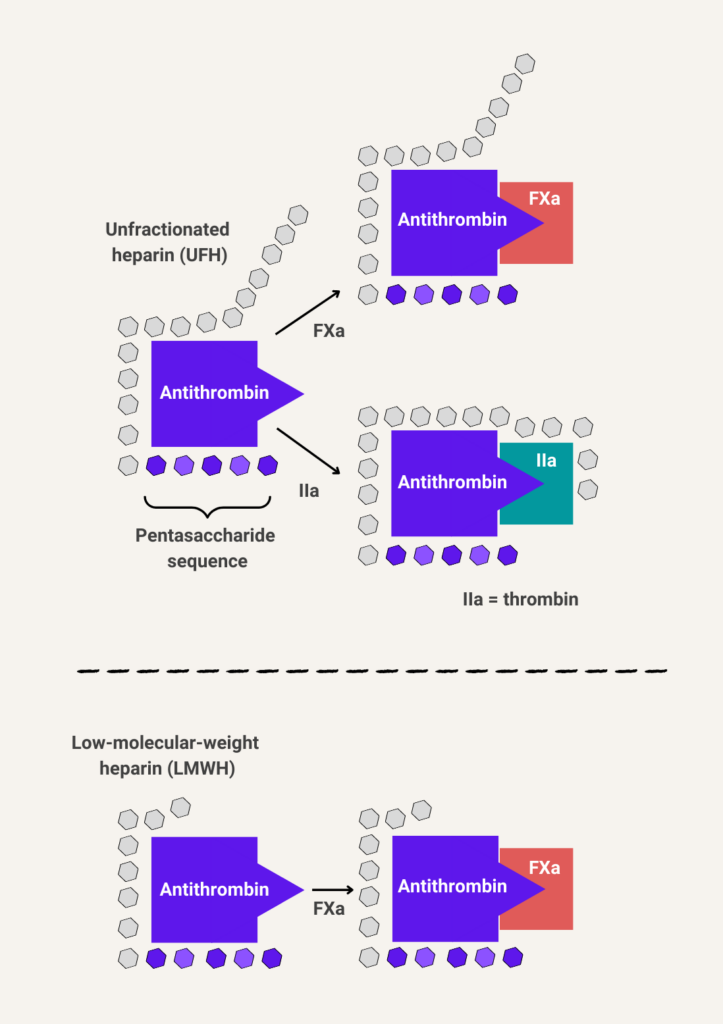
LMWH is derived from full-length, highly sulfated polysaccharide (unfractionated heparin) harvested from porcine mucosal tissue by chemical or enzymatic depolymerization, resulting in smaller size polysaccharide fragments (6-22 sugar residues). Heparin binds to antithrombin through a pentasaccharide region leading to the inhibition of thrombin and Factor Xa (FXa). . Only one third of heparin polysaccharide chains display anticoagulant properties implying that a specific sequence in the polysaccharide chain is required for this activity. The number of polysaccharide fragments determines which serine proteases are inactivated by antithrombin. Inactivation of thrombin requires that the heparin be long enough (> 18 sugar residues) to bind both antithrombin and thrombin, serving as a bridge between the protease and its inhibitor. By contrast, inactivation of FXa simply requires the pentasaccharide sequence to bind to antithrombin (there is no need for bridging FXa with antithrombin). The majority of LMWH is shorter than this threshold. Thus, most of its activity is exerted through factor Xa inhibition.
Related FAQs:
- What is the half-life of low-molecular-weight heparin (LMWH)?
- Is low-molecular-weight heparin (LMWH) safe in patients with chronic liver disease/cirrhosis?
- How do I use protamine sulfate to reverse low-molecular-weight heparin (LMWH)?
- Does treatment of venous thromboembolism (VTE) with low-molecule-weight heparin (LMWH) require one or two doses per day?
- What is the renal clearance of LMWH?
- What does LMWH stand for?
- How is low-molecular-weight heparin (LMWH) monitored?
