I posted the following poll about whether you would provide thromboprophylaxis in a patient with a past history of a long-haul VTE who is not undergoing in vitro ferritization.
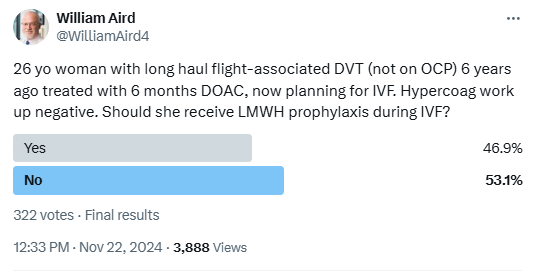
There was almost a 50:50 split in responses.
Bottom line
- VTE is more common with IVF compared with non-IVF pregnancies.
- VTE is particularly prevalent in patients undergoing IVF who develop ovarian hyperstimulation syndrome (OHSS).
- There is little evidence to support treatment decisions in this patient population.
- There are 2 clinical practice guidelines that provide guidance on thromboprophylaxis in this setting.
- According to Swedish Association of Obstetrics and Gynecology, our patient would have received 4 points for her previous VTE and thus would have been recommended to receive thromboprophylaxis with LMWH once daily throughout pregnancy and at least for 6 weeks postpartum (thromboprophylaxis twice daily (=double dose) throughout), commenced at the initiation of FSH/HMG or estrogen stimulation.
- According to the Royal College of Obstetricians and Gynecologists, our patient would have scored 4 points for her previous VTE, and therefore would have been recommended to receive thromboprophylaxis with LMWH in the first trimester.
Introduction
- In1978, Robert Edwards announced the birth of the first test tube baby .1
- Today, approximately 5 million babies are born after in vitro fertilization (IVF).
- A relationship between ovarian stimulation and thrombosis was first suggested in 1965.2
- The incidence of VTE has been reported as 0.1%–0.5% of all IVF cycles.3
- A recent systematic review has confirmed that the antepartum risk of VTE after IVF is doubled (odds ratio, 2.18; 95% confidence interval, 1.63–2.92) in comparison with the background normal pregnant population. 4
- The risk of arterial thrombosis is much lower; nearly 70% of reported cases are venous thrombosis and 30% are arterial. 56
- Thrombosis connected to IVF has been shown to have a propensity to occur in the upper extremities.
Definitions
- Infertility is the inability to achieve a pregnancy after 12 months of regular, unprotected sexual intercourse.7
- Artificial reproductive therapy (ART):8
- Includes all fertility treatments in which either eggs or embryos are handled.
- In general, ART procedures involve surgically removing eggs from a woman’s ovaries, combining them with sperm in the laboratory, and returning them to the woman’s body or donating them to another woman. C
- They do NOT include treatments in which only sperm are handled (i.e., intrauterine—or artificial—insemination) or procedures in which a woman takes medicine only to stimulate egg production without the intention of having eggs retrieved.
- In vitro fertilization:
- In vitro fertilization (IVF) involves the extraction of eggs, fertilization in the laboratory, and transfer of embryos into the uterus through the cervix.9
- During IVF, eggs and sperm from the couple are incubated together in a laboratory to produce an embryo. A health care provider then places the embryo into the woman’s uterus, where it may implant and result in a successful pregnancy.10
- IVF involves a doctor extracting eggs and fertilizing them in a special lab. Specialists can combine this with an embryo transfer (IVF-ET) and transfer the resulting embryos into a person’s uterus. The Society for Assisted Reproductive Technology states that IVF-ET accounts for 99% of ART procedures. (NIH)
- Superovulation refers to the use of pharmacologic treatments to induce ovulation, and ovarian stimulation, which is performed with the goal of inducing multiple mature ovarian follicles. 11
- Ovarian hyperstimulation syndrome:12
- Ovarian hyperstimulation syndrome (OHSS) is a complication of fertility treatment, which uses pharmacological ovarian stimulation to increase the number of oocytes and therefore embryos available during assisted reproductive technology (ART).
Classification
- Primary infertility is having never achieved a pregnancy. Secondary infertility is the inability to achieve a pregnancy after a previous pregnancy.13
Epidemiology
- Infertility:
- Infertility affects between 8% and 12% of couples of reproductive age worldwide.14
- In the United States, 12.2% of females 15 to 49 years of age have received infertility services.15
- Globally, 48 million couples and 186 million individuals are affected by infertility.16
- Infertility affects 8.8% of US women aged 15 to 49 years 17
- Assisted reproductive technology (ART) now accounts for 1.6% of all infants and 18.3% of all multiple-birth infants in the United States.18
- Moderate or severe ovarian hyperstimulation syndrome (OHSS) which affects 2–3% of patients, as milder forms may develop in 20–30% of all in-vitro fertilization (IVF) cycles, and it is the moderate/severe OHSS which incurs risk of acute renal insufficiency, acute respiratory distress syndrome and venous thromboembolism.19
- Thrombosis occurring as a complication of IVF:
- In 2012, Rova et al. reported on the risk of VTE in early pregnancy in relation to IVF and OHSS. The review included all deliveries in Sweden (n = 964,532) during the period 1999–2008. Of these, 19,162 were IVF pregnancies compared to 935,178 non-IVF pregnancies. The incidence of VTE in the first trimester:20
- In non-IVF pregnancies was 0.2 per 1000 women
- In IVF pregnancies with no OHSS was 0.8 per 1000 women (OR 4.8, 95% CI 2.7–8.7)
- In IVF pregnancies with OHSS 16.8 VTE events per 1000 women (OR 99.7, 95% CI 61.6–161.1).
- Gurunath et al:
- The prevalence of venous thromboembolism (VTE) in patients undergoing assisted reproduction (0.1–0.2%) is ten times higher than the general population (2.2/10,000) though the absolute numbers are low.
- Ovarian hyperstimulation syndrome (OHSS) increases the risk of thrombosis dramatically to 100-fold or an absolute risk of 1.7%.21
- Systematic review:22
- The frequency of TE during pregnancy in patients after IVF, with or without OHSS varies between 0.8 and 25/1000, compared with 0.17–2.5/1000 in the background pregnant population.
- In studies assessing the risk of antepartum VTE, the reported risk was approximately doubled, odds ratio (OR) 2.2 (95% CI 1.6–2.9).
- Reported risk of first-trimester VTE was increased 5- to 10-fold (OR 6.4, 95% CI 4.0–10.1).
- The risk of VTE after IVF failing to lead to conception was not increased compared with a reference population.
- ATE was rare and a high propensity for ATE in relation to OHSS was reported.
- SFOG (Swedish Association of Obstetrics and Gynecology):
- The incidence of venous thrombosis during spontaneous pregnancy is estimated to 1/1000 pregnancies, and after IVF to 2/1000. The risk of thrombosis in the first trimester in IVF pregnancy increases by 5-10 times, mainly due to an up to 100-fold risk increment during ovarian hyperstimulation syndrome (OHSS) in IVF pregnancies that result in born babies.
- There are approximately 19,000 IVF treatments performed annually in Sweden. The risk of both arterial and venous thrombosis increases with ovarian hyperstimulation syndrome (OHSS). The risk of thrombosis in pregnancy decreases by 85-90 % when recommended thromboprophylaxis is used.
- The risk of VTE may be increased in women with obesity, multiple pregnancy, smoking, advanced age, hyperhomocysteinemia, and cesarean section.23
- In 2012, Rova et al. reported on the risk of VTE in early pregnancy in relation to IVF and OHSS. The review included all deliveries in Sweden (n = 964,532) during the period 1999–2008. Of these, 19,162 were IVF pregnancies compared to 935,178 non-IVF pregnancies. The incidence of VTE in the first trimester:20
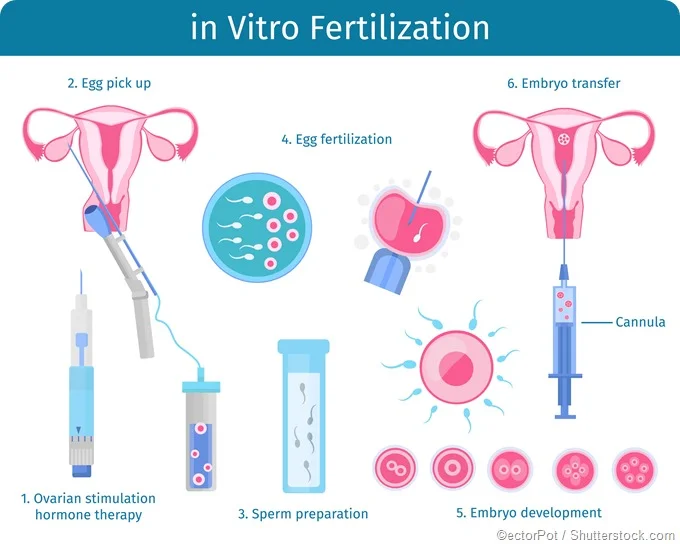
In vitro fertilization steps
- Superovulation:
- Also known as ovarian stimulation, ovulation induction, or stimulation of egg maturation.
- Without stimulating medications, the ovaries make and release only 1 mature egg per menstrual cycle (month). Hyperstimulation of the ovaries is done to allow the release of more than one ovum. This step lasts an average of 8–12 days and may include medications like Follistim, Menopur, Gonal-F, Bravelle, Repronex, or a combination.
- Three basic elements:
- Exogenous gonadotrophins to stimulate multi‐follicular development:
- Human menopausal gonadotrophin (hMG), A urinary product with follicle‐stimulating hormone (FSH) and luteinizing hormone (LH) activity
- Purified FSH (p‐FSH) and highly purified FSH (hp‐FSH)
- Various recombinant FSH (rFSH) and LH (rFSH/rLH) preparations
- Cotreatment with either gonadotropin‐releasing hormone (GnRH) agonist or antagonists to suppress pituitary function and prevent premature ovulation:
- GnRH antagonists act by binding to the GnRH receptors and prevent endogenous release of GnRH from the pituitary gland. GnRH antagonist protocols are associated with immediate LH suppression and decreased gonadotrophin use.
- Triggering of final oocyte maturation 36 to 38 hours prior to oocyte retrieval. At the end of the stimulation phase of an IVF cycle, a drug is used to trigger the final oocyte maturation, which is used to mimic the natural endogenous LH surge and initiate the process of ovulation before the mature eggs are collected from the woman and fertilized with sperm in the laboratory. Two drugs are currently used:
- Human chorionic gonadotropin (HCG), which is the most common drug
- GnRH agonist in an antagonist protocol
- Exogenous gonadotrophins to stimulate multi‐follicular development:
- Egg Retrieval:
- This is the process used to remove the eggs from the ovaries so they can be fertilized. The procedure is performed in a physician’s office as an outpatient procedure. A mild sedative and painkiller are often used during the procedure, and it normally takes about 30 minutes. The steps for egg retrieval are as follows:
- An ultrasound probe is inserted into the vagina to visualize the ovaries and the follicles, which contain the eggs.
- A needle is inserted through the wall of the vagina to the ovaries. Generally, ultrasound is used to guide the placement of the needle.
- Suction is used to pull the eggs from the ovaries into the needle.
- This is the process used to remove the eggs from the ovaries so they can be fertilized. The procedure is performed in a physician’s office as an outpatient procedure. A mild sedative and painkiller are often used during the procedure, and it normally takes about 30 minutes. The steps for egg retrieval are as follows:
- Fertilization:
- A man provides a semen sample. If the sperm are healthy, they are centrifuged to concentrate them and reduce the volume, placed in a dish with the egg, and left overnight in an incubator.
- Fertilization usually occurs on its own. However, sometimes sperm are not able to fertilize the egg on their own.
- When this is the case, a single sperm is injected into an egg using a needle.
- This process is called intracytoplasmic sperm injection (ICSI).
- About 60% of IVF in the Unites States is performed with ICSI.
- The pregnancy rate is about the same for IVF using natural fertilization or ICSI
- Embryo Transfer:
- This procedure is performed in a physician’s office. The procedure is normally painless, but some women may experience cramping.
Ovarian hyperstimulation syndrome
- All ovulation induction medications confer a risk of ovarian hyperstimulation syndrome and multifetal gestation.24
- Ovarian hyperstimulation syndrome manifests as:25
- Abdominal pain and distention
- Ascites
- Gastrointestinal problems
- Respiratory compromise
- Oliguria
- Hemoconcentration
- Thromboembolism
- Most common with the use of gonadotropins and high-dose oral ovulation induction medications.26
- Exposure of ovaries to human chorionic gonadotrophin (hCG) or luteinizing hormone (LH) following controlled ovarian stimulation by follicle-stimulating hormone (FSH) underlies most cases of OHSS.27
- Exposure of hyperstimulated ovaries to hCG leads to the production of proinflammatory mediators, especially vascular endothelial growth factor (VEGF) (in response to (hCG, VEGF mediates the vascular permeability of OHSS the cardinal feature of the syndrome), which causes increased vascular permeability, leading to loss of fluid into the third space, manifesting as ascites or, less commonly, pleural and pericardial effusions.
- Women with severe OHSS demonstrate hypovolemia, with a typical loss of 20% of their calculated blood volume in the acute phase of OHSS. Accompanying this hypovolemia is reduced serum osmolality and sodium. This paradoxical combination of hypovolemia and hypo-osmolality has been ascribed to a ‘reset’ of the osmotic thresholds of vasopressin and thirst to lower osmolality and sodium levels as these women remain able to concentrate and dilute their urine around the new, lower, level of osmolality.28
- Severe OHSS is a prothrombotic state due to hemoconcentration and vascular endothelial dysfunction. The incidence of thrombosis been estimated to lie between 0.7% and 10% of cases of OHSS.
- Ovarian hyperstimulation syndrome is treated with supportive care, including antiemetics, volume replacement, and, in severe cases, paracentesis. Patients should be counseled on these risks before treatment.29
Complications of IVF
- Complications include:30
- Multiple delivery
- Preterm delivery
- Low birth-weight delivery experienced with ART
- Ovarian hyperstimulation syndrome (OHSS)
- VTE especially with OHSS
- Doubling in the risk for severe maternal morbidity even after controlling for maternal age, parity, and comorbid conditions
- Factors that may contribute to the adverse effects of IVF on pregnancy outcomes include:31
- Those related to the IVF procedure itself:
- Medications
- Laboratory conditions during embryo culture
- Culture medium
- Cryopreservation, and thawing
- Maternal conditions associated with subfertility and infertility including:
- Pregnancy at an older age
- Reduced ovarian reserve
- Those related to the IVF procedure itself:
Thrombosis associated with IVF
- Epidemiology (discussed above under Epidemiology, elaborated on here):
- IVF is reported to double the risk of TE in pregnancy, but the absolute risk is presumed to be low.
- Systematic review:32
- Total of 21 articles included.
- The frequency of TE during pregnancy in patients after IVF, with or without OHSS varies between 0.8 and 25/1000, compared with 0.17–2.5/1000 in the background pregnant population.
- In studies assessing the risk of antepartum VTE, the reported risk was approximately doubled, odds ratio (OR) 2.2 (95% CI 1.6–2.9) (Figure 2)(5,11,13,14,17) and the reported risk of first-trimester VTE was increased 5- to 10-fold.
- The risk of VTE after IVF failing to lead to conception was not increased compared with a reference population.
- The reported interval from embryo transfer (ET) to VTE was 3–112 day.
- 40% of patients had coexisting thrombophilia
- We conclude that the antepartum risk of VTE after IVF is approximately doubled, mainly due to a 5- to 10-foldincreased risk during the first trimester, in turn primarily due to a very high risk in the subgroup complicated by OHSS.
- Thrombosis connected to IVF has been shown to have a propensity to occur in the upper extremities
- A suggested explanation for the increased risk of upper-extremity TE is the result of inflammatory peritoneal fluid draining through the thoracic ducts.
- Risk factors:
- Risk factors for enhancing the probability of thrombosis in IVF include:33
- Aggressive stimulation
- Elevated estradiol levels
- Use of hCG as a trigger
- OHSS
- Occurrence of pregnancy
- Multiple pregnancy
- Use of exogenous estrogen for FET
- Risk factors for enhancing the probability of thrombosis in IVF include:33
- Mechanisms:
- Hypoestrogenism that results from ovarian stimulation causes:
- Increase in procoagulant factors (fibrinogen, von Willebrand factor, factors VIII and V, and increased activated protein C resistance
- Enhanced activation of coagulation (D-dimer)
- Reduced fibrinolysis (reduced tissue plasminogen activator and plasminogen activator inhibitor type I)
- Reduced natural anticoagulants (antithrombin III and protein S)
- Hypoestrogenism that results from ovarian stimulation causes:
- Timing:34
- It is rare to experience VTE before administration of hCG. Of over 100 reported cases of thrombosis in IVF, only 1 case was before the administration of hCG. Only 3% of both arterial and VTE have been known to occur before final oocyte maturation trigger.
- Thromboprophylaxis:
- Systematic review:35
- Thromboprophylaxis was reported on in three studies; only one study including 24 women had this as the primary aim.
- Heparin (LMWH) with or without aspirin was administered in all reported studies, but mostly the type and dose were not stated.
- Despite administered prophylaxis, the number of TE cases was higher than expected in a normal pregnant population.
- Expert opinion:
- Gurunath et al:36
- Not indicated in all patients without any risk factors.
- All women should be assessed for risk of VTE as per the Royal College of Obstetricians and Gynecologists or Swedish guidelines before commencement of treatment, and dose and duration of thromboprophylaxis must be decided in consultation with a hematologist.
- Wherever thromboprophylaxis is indicated during pregnancy, it should be commenced at the start of stimulation.
- Patients with a history of prior VTE, antiphospholipid syndrome (APAS) without VTE or those with multiple risk factors are at high risk for thrombosis and require thromboprophylaxis during IVF stimulation and pregnancy.
- Patients who are very high risk for thrombosis include those with mechanical heart valves, VTE with APAS, recurrent VTE, or antithrombin deficiency.
- Gurunath et al:36
- Clinical practice guidelines:
- SFOG (Swedish Association of Obstetrics and Gynecology):
- Thromboprophylaxis is not indicated in patients without known risk factors undergoing IVF treatment without complications.
- Pregnant patients diagnosed with OHSS should continue thromboprophylaxis until resolution of OHSS, and at least until gestational week 12+6. If additional risk factors are present, thromboprophylaxis should be continued according to the scoring system in the HEM-ARG guidelines.
- Thromboprophylaxis can be discontinued 4 weeks after resolution of OHSS in patients that are not pregnant.
- In patients where thromboprophylaxis is indicated during pregnancy this should be commenced at the initiation of FSH/HMG or estrogen stimulation.
- A preconceptional evaluation and decision regarding thromboprophylaxis (risk score ≥2, according to score system below) during IVF stimulation and pregnancy is recommended.
- An individual plan for dosing of thromboprophylaxis (normal dose or high dose) is essential, especially so for patients with “very high risk” of thrombosis. An individual treatment plan should always be prepared in collaboration with an obstetrician experienced in coagulation treatment, prior to IVF.
- Scoring system:
- 1 point:
- 2 points:
- Protein S deficiency
- Protein C deficiency
- Immobilization40
- 3 points:
- Hom FV Leiden
- Hom prothrombin
- More than one thrombophilic defect
- 4 points:
- Prior VTE
- APS without VTE41
- OHSS
- Very high risk:
- Mechanical aortic valve
- Condition warranting continuous thromboprophylaxis
- APS with VTE
- Recurrent VTE
- Antithrombin deficiency
- Planning of thromboprophylaxis: management based on clinical score:
- A preconceptional evaluation and decision regarding thromboprophylaxis (risk score ≥2, according to Summary Box 1) during IVF stimulation and pregnancy is recommended.
- Treatment according to score:
- 1 point: thromboprophylaxis not needed
- 2 points: thromboprophylaxis postpartum once daily for at least 7 days, this includes thromboprophylaxis for a transient risk factor
- 3 points: thromboprophylaxis once daily for 6 weeks postpartum
- 4 or higher points: thromboprophylaxis once daily throughout pregnancy* and at least for 6 weeks postpartum
- Very high risk: thromboprophylaxis twice daily (=double dose) throughout pregnancy* and at least for 12 weeks postpartum
- Action plan for thromboprophylaxis for patients with conditions entailing very high risk of thromboembolic complications:
- Recurrent VTE, ongoing oral anticoagulation therapy and possibly patients with sequelae after previous TE: High dose prophylaxis LMWH is initiated prior to conception or as soon as pregnancy is confirmed and is continued at least 6 weeks postpartum or until recommencement of previous treatment
- Hereditary antithrombin deficiency:
- High dose prophylaxis LMWH is initiated prior to conception or as soon as pregnancy is confirmed and is administered according to individual treatment plan.
- Antithrombin concentrate if complications and at delivery.
- APS with TE: Normal dose prophylaxis LMWH + ASA 75 mg x 1 is initiated prior to conception or as soon as pregnancy is confirmed, and continued at least until 12 weeks postpartum.
- Ovarian hyperstimulation syndrome: Normal dose prophylaxis LMWH is given during the entire first trimester and until resolution of symptoms
- Royal College of Obstetricians and Gynecologists:42
- Women with an IVF pregnancy and three other risk factors [the guideline refers to risk factors, not to risk score] should be considered for thromboprophylaxis. with LMWH starting in the first trimester. Risk factors include:
- One point:
- Family history of unprovoked or estrogen-related VTE in first-degree relative
- Known low-risk thrombophilia (no VTE)
- Age (> 35 years)
- Obesity
- Parity ≥ 3
- Smoker
- Gross varicose veins
- 3 points:
- Previous DVT provoked by surgery
- Known high-risk thrombophilia
- Medical comorbidities e.g. cancer, heart failure; active systemic lupus erythematosus, inflammatory polyarthropathy or inflammatory bowel disease; nephrotic syndrome; type I diabetes mellitus with nephropathy; sickle cell disease; current intravenous drug user
- 4 points: Previous VTE (except provoked by major surgery)
- One point:
- Women with ovarian hyperstimulation syndrome should be considered for thromboprophylaxis with LMWH in the first trimester.
- Women with an IVF pregnancy and three other risk factors [the guideline refers to risk factors, not to risk score] should be considered for thromboprophylaxis. with LMWH starting in the first trimester. Risk factors include:
- Royal College of Obstetricians and Gynecologists:43
- Women with severe or critical OHSS and those admitted with OHSS should receive LMWH prophylaxis.
- The duration of LMWH prophylaxis should be individualized according to patient risk factors and outcome of treatment.
- Women with moderate OHSS should be evaluated for predisposing risk factors for thrombosis and prescribed either antiembolism stockings or LMWH if indicated.
- In addition to the usual symptoms and signs of venous thromboembolism (VTE), thromboembolism should be suspected in women with OHSS who present with unusual neurological symptoms, even if they present several weeks after apparent improvement in OHSS.
- In women with severe OHSS who conceive, thromboprophylaxis should be considered at least until the end of the first trimester
- SFOG (Swedish Association of Obstetrics and Gynecology):
- Systematic review:35
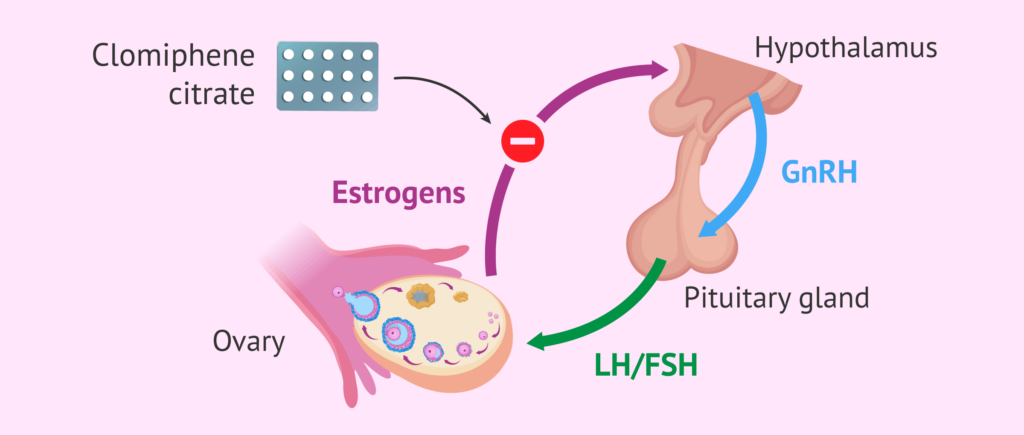
Its mode of action is based on its similarity to estrogens. That is why clomiphene citrate binds to estrogen receptors found in the hypothalamus, competing for those receptors with estrogen and thus reducing their action. This binding promotes the stimulation of and secretion of GnRH, which in turn produces the release of FSH and LH gonadotropins.
When treatment with clomiphene citrate is suppressed, a decrease in GnRH is produced, which causes estradiol to increase 2 or 3 times its normal amount. The increase in estradiol stimulates the LH peak that will trigger ovulation. That is why its uncontrolled use can generate the risk of multiple gestation.