I posted a poll on Twitter on 3/12/24 asking whether a patient with sickle cell disease in pain crisis, and an SaO2 of 96%, should receive supplemental oxygen.
Most respondents answered YES.
- There is no evidence to support the use of oxygen supplentation in a patient with sickle cell disease (SCD) whose SaO2 is already in a healthy range.
- But what is the threshold for a healthy SaO2?
- All recommendations regarding the use of supplemental oxygen in patients with SCD are opinion-based.
- Several physiological principles are worthy of consideration.
Bottom line: Providing supplemental oxygen in an individual whose SaO2 is high provides (e.g., > 95%) little increment in oxygen delivery, while increasing the PaO2 (hyperoxia). Supplemental oxygen therapy resulting in hyperoxia is not risk-free. Hyperoxia may result in increased production of reactive oxygen species (ROS) which may then cause tissue damage, especially in the lung. In patients with sickle cell disease (SCD), there are added risks of suppressing erythropoiesis, thus contributing to anemia, and rebound pain when the supplemental oxygen is stopped. Though there is no evidence to support a conclusion one way or another, a consideration of first principles seems to favor the avoidance of supplemental oxygen in patient with SCD whose SaO2 is > 95%.
Physiological principles
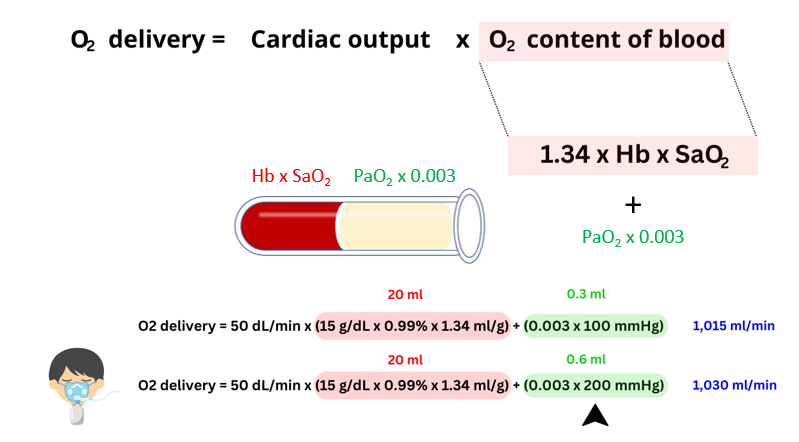
- Increasing the PaO2 with supplemental oxygen in a patient whose SaO2 is already high has little effect on oxygen content of blood:
- The vast majority of oxygen in the blood is bound to hemoglobin; only a tiny amount is dissolved in plasma.
- Once the Hb is fully saturated with oxygen (i.e. 100%), any further increase in PaO2 (i.e. with supplemental O2) will serve only to (barely) augment the amount of dissolved oxygen while creating a state of hyperoxia.
- In patients with sickle cell disease:
- The prevalence of hypoxemia during steady-state, defined as transcutaneous SpO2 less than 96%, has been reported to range between 33%-44%. Causes include:1
- Acute chest syndrome causing ventilation/perfusion mismatch secondary to pneumonia, atelectasis, and in severe episodes, adult respiratory distress syndrome.
- Elevated levels of dyshemoglobins, carboxyhemoglobin and methemoglobin.2
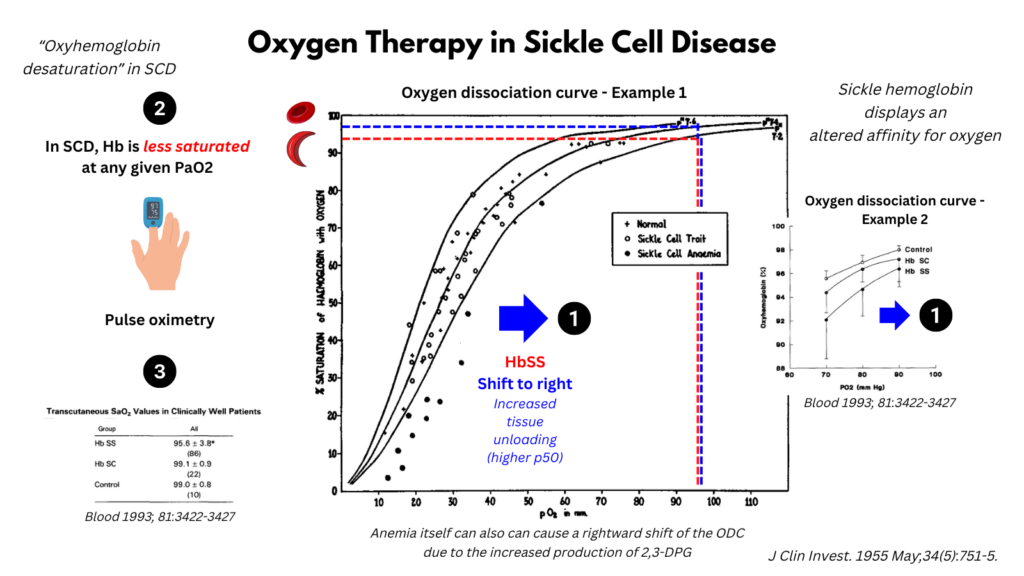
- The oxygen dissociation curve is shifted to the right,
- Caused by:
- Decreased oxygen affinity of HbS
- Anemia per so, due to the increased production of 2,3-DPG
- Leading to:
- Increased unloading of oxygen in the peripheral tissues.
- Relative oxygen desaturation, meaning that for any given PaO2, the SaO2 is lower.
- Difficulty achieving a SaO2 higher than 95-96% even with a PaO2 around 100 mg Hg.
- Caused by:
- The prevalence of hypoxemia during steady-state, defined as transcutaneous SpO2 less than 96%, has been reported to range between 33%-44%. Causes include:1
- Potential risk-benefits of hyperoxia:
- Benefits:
- In patients with SCD:
- Reduction in irreversibly sickled cells.
- In patients with SCD:
- Risks:
- General:
- Increased ROS production, resulting in:
- Damage to DNA, proteins and lipids
- Organ dysfunction, especially pulmonary
- Hyperoxia can induce pro- and anti-inflammatory responses
- Respiratory stimulant resulting in hypocapnia:
- Vasoconstriction
- Increased affinity of hemoglobin for O2
- Increased ROS production, resulting in:
- In patients with SCD:
- Suppression of erythropoiesis with resultant bone marrow suppression and anemia.
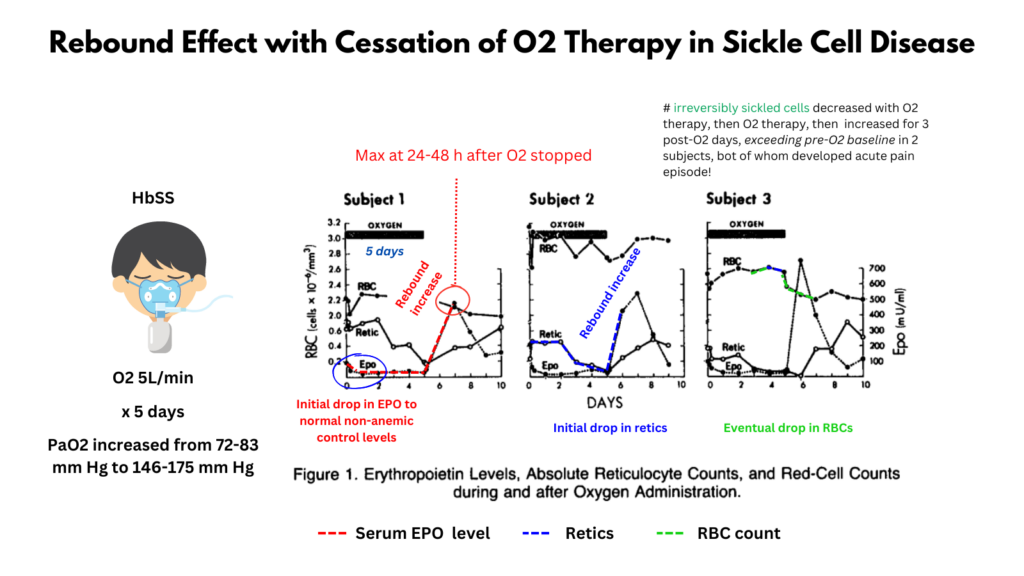
- Cessation of oxygen therapy in patients with SCD may result in rebound increase in erythropoiesis and irreversibly sickled cells:3
- Production of erythropoietin (EPO) is mediated by the PaO2, not the SaO2; thus hyperoxia results in suppression of EPO levels.
- Though the number of irreversibly sickled cells has not been shown to correlate with acute complications in SCD, two of the three subjects in the study below presented with rebound pain following cessation of oxygen therapy.4
- General:
- The following are excerpts from papers discussing the role of PaO2 vs SaO2 in mediating erythropoietin production, as well the risk-benefits of oxygen-induced hyperoxia in general, and more specifically in patients with sickle cell disease:
- Benefits:


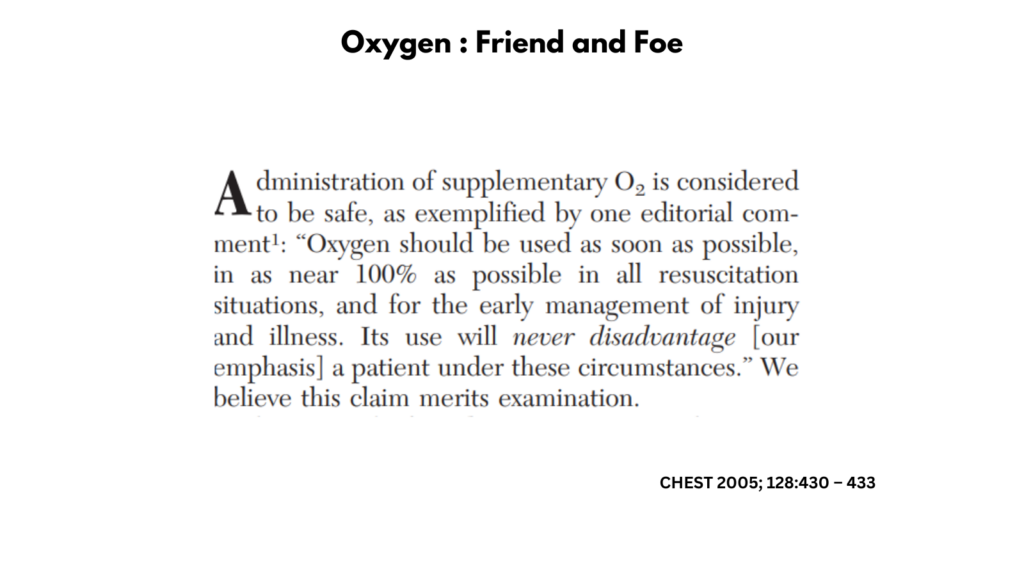

Clinical Practice Guidelines5
- NIH Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014

- British Society for Haematology:
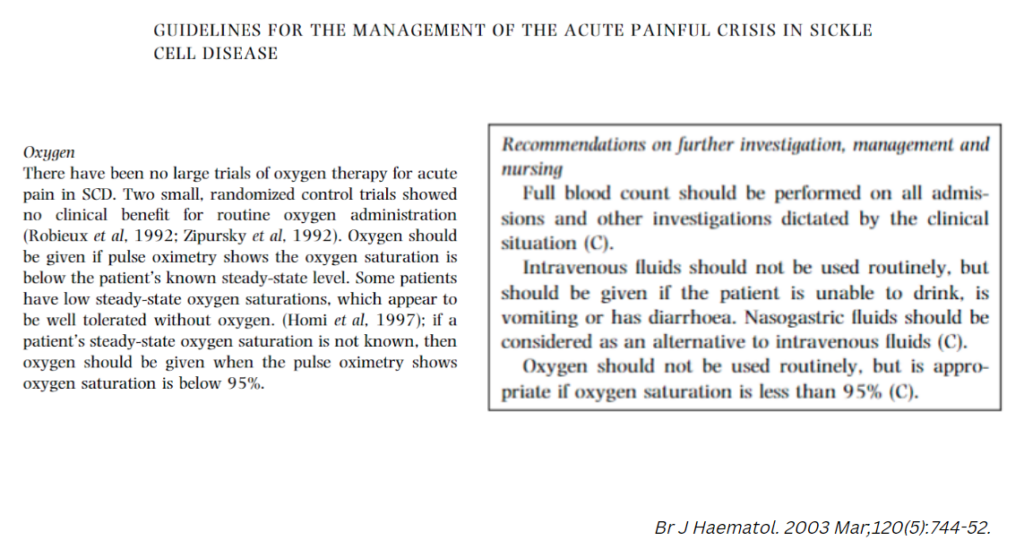
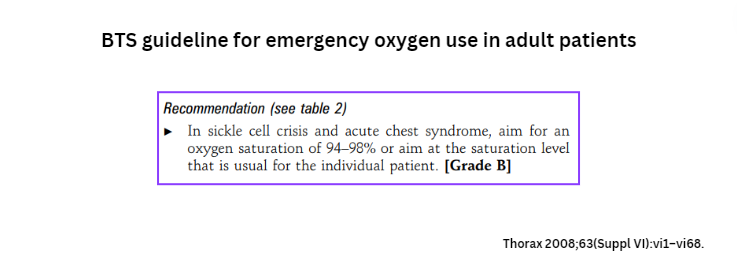
- British Thoracic Society Guideline for oxygen use in adult patients