Overview of ferritin
Ferritin is the body’s primary iron storage protein, safely sequestering iron inside cells to prevent toxicity while making it available when needed. Ferritin is found in nearly all cells of the body, especially those involved in iron storage and metabolism, such as hepatocytes, macrophages, and enterocytes. Inside cells, ferritin functions as a safe iron reservoir, capable of storing up to 4,500 iron atoms per molecule in a bioavailable and non-toxic form. This intracellular ferritin helps regulate iron homeostasis, protects against oxidative stress, and ensures iron is available for critical processes like hemoglobin synthesis and mitochondrial respiration.
Ferritin is a 24-subunit protein composed of two types of chains: heavy (H) and light (L). The H chain has ferroxidase activity, enabling it to convert ferrous iron (Fe²⁺) to ferric iron (Fe³⁺), facilitating safe iron storage. It is most abundant in tissues with high metabolic demand for iron regulation, such as the heart and brain. The L chain, lacking enzymatic activity, promotes iron nucleation and long-term storage and is predominant in iron storage organs like the liver and spleen.
Serum Ferritin
Serum ferritin, on the other hand, is a small fraction of total body ferritin and primarily reflects the iron content of intracellular stores. It is thought to be secreted mainly by macrophages and hepatocytes. However, the mechanism of secretion is atypical: it occurs through non-classical secretory pathways, not the standard endoplasmic reticulum-Golgi route.
Importantly, serum ferritin is mostly iron-poor, contains mostly L chains, and does not directly function in iron transport or delivery (its biological function remains unclear). Its primary role is as a clinical biomarker, providing an indirect measure of body iron stores. However, because ferritin expression is upregulated by inflammatory cytokines (e.g., IL-6), serum ferritin levels can also rise in the setting of inflammation, infection, malignancy, or liver disease, independent of iron status. This dual regulation by iron levels and inflammation is what makes ferritin both useful and sometimes challenging to interpret in clinical practice.
Regulation of Ferritin Levels
- Translational control by iron:
- Ferritin synthesis is iron-responsive at the translational level.
- When intracellular iron is low, iron regulatory proteins (IRPs) bind to the iron-responsive element (IRE) on ferritin mRNA, blocking translation.
- When iron is high, IRPs dissociate, allowing translation to proceed → more ferritin protein is made to safely store excess iron.
- This mechanism ensures cells only make ferritin when there’s iron to store.
- Transcriptional control by inflammation:
- Cytokines (especially IL-1 and IL-6) stimulate transcription of ferritin genes.
- This happens via activation of acute-phase pathways (e.g., STAT3 pathway with IL-6), leading to increased ferritin mRNA production regardless of iron status.
- This explains why ferritin rises in inflammation, even when iron stores are low (as in anemia of chronic disease).
- Ferritin release into serum:
- Serum ferritin comes from intracellular ferritin released into the plasma, mostly from the liver and macrophages.
- It may also reflect cellular leakage in cases of liver injury or generalized cell turnover.
- Serum ferritin is mostly iron-poor, unlike intracellular ferritin, which is iron-rich.
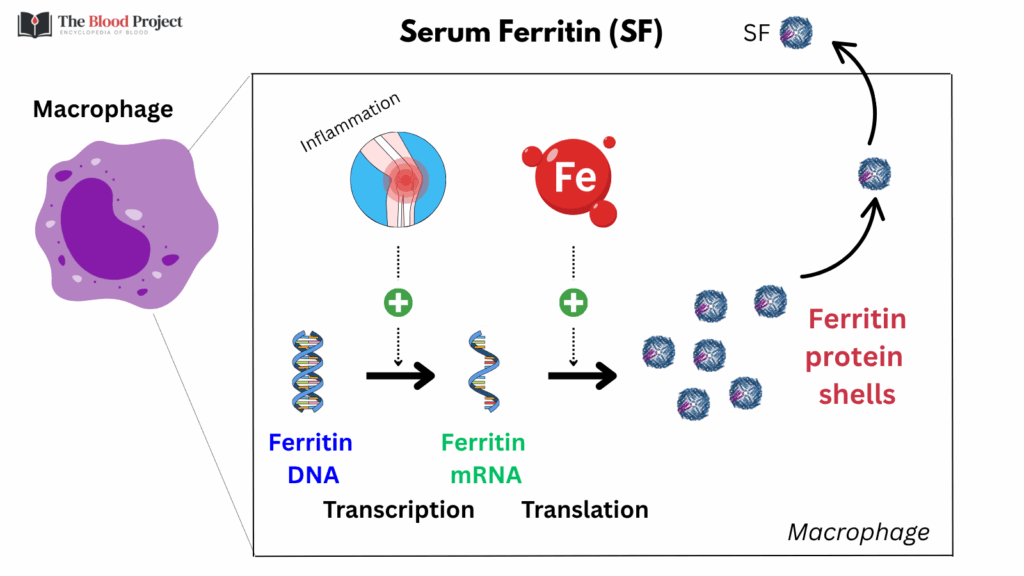
For larger image, click here.
Why iron deficiency limits ferritin elevation in inflammation:
In patients with inflammation (e.g., chronic disease, infection), a serum ferritin <30 ng/mL is diagnostic of absolute iron deficiency, and a ferritin >100 ng/mL virtually rules it out. That’s because iron deficiency limits the extent to which ferritin can increase, no matter how inflamed the patient is.
- Inflammation tries to increase ferritin mRNA.
- But iron deficiency blocks ferritin translation, so protein production remains limited.
- The result is that serum ferritin cannot rise very much, even in the presence of strong inflammatory signals.
The translational block wins out over the transcriptional push. This molecular tug-of-war is the key reason why serum ferritin remains a reliable marker of iron stores, even in the context of systemic inflammation.
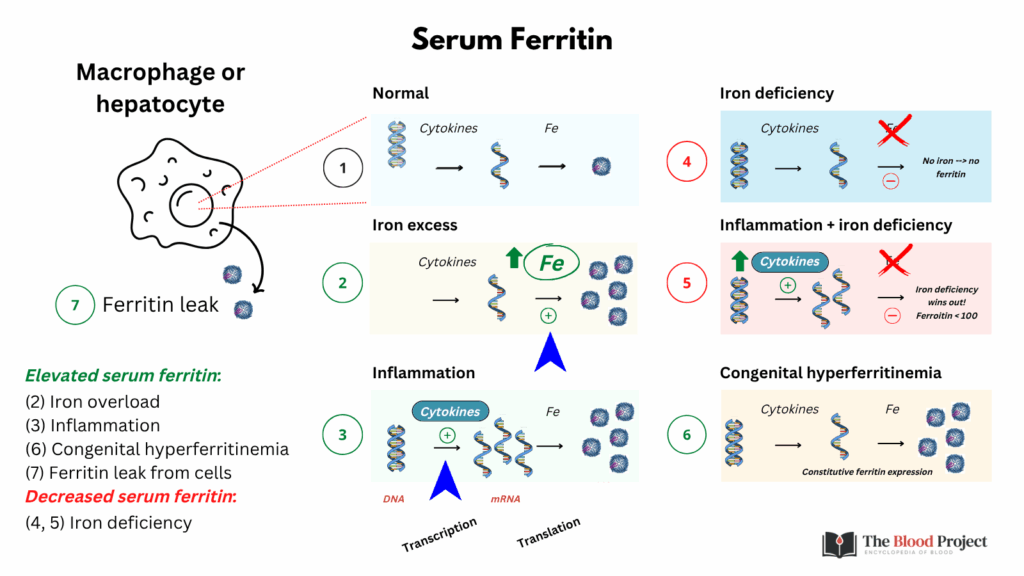
For larger image, click here.
