I posted a poll asking what the absolute nucleated red blood cell (nRBC) was based on the CBC below. See graphic for the correct answer and explanation.

Overview
- Nucleated red blood cells (nRBCs) (aka normoblasts, erythroblasts) are immature erythrocytes that are normally present in the peripheral blood of neonates but usually disappear in the first few days of life.12
- College of American Pathologists: “The term nucleated red blood cells is used to state the presence of normoblasts in the peripheral blood and includes all normoblasts regardless of the stage of maturation.”
- The presence of circulating nRBCs in healthy children and adults is considered an abnormal finding and possible harbinger of underlying disease.3
- When seen in peripheral blood, nRBCs should be enumerated and reported as number of nRBCs per 100 white cells or as an absolute count.
- Reports in hospitalized patients show that nRBCs are an indicator of poor prognosis and increased mortality, especially in critical care settings.4
History
- nRBCs were first described in peripheral blood by Paul Ehrlich in 1880, shortly after his introduction of staining methods in hematology. He wrote a paper, “Findings in Anemia-De- and Regeneration of Blood Cells,” in which he differentiated between the “normoblasts” present in the anemia of blood loss and the “megaloblasts” present in pernicious anemia.5
- The term erythroblastemia was used in the past to describe the presence of nucleated red cells in circulating blood.
- The first clinical application of nRBCs counts was published in 1969.6

Mechanisms
- Erythrocyte differentiation involves a multistep pathway that begins with the hematopoietic stem cell and ends with the mature RBC.
- Adult mammalian erythrocytes are anucleate (they lack a nucleus); this has several advantages:
- The cell can assume a biconcave shape, which increases its surface-to-volume ratio:
- Allowing passage through narrow capillaries (increased deformability).
- Optimizing oxygen diffusion.
- Anucleate red cells lack mitochondria, so they do not consume the oxygen they are carrying to other tissues.
- These cells can pack in more hemoglobin (since they do not have space occupying nucleus), thus facilitating oxygen delivery.
- The cell can assume a biconcave shape, which increases its surface-to-volume ratio:
- Enucleation, the process by which the nucleus is removed from red blood cells, is the penultimate step in mammalian erythroid terminal differentiation (the final step involves differentiation of reticulocytes into mature RBCs).7
- Once blood cells are ready for release into the circulation they pass through fenestrae (holes) in the sinusoidal endothelium of the bone marrow. Normal mature bone marrow cells are deformable, so they can squeeze through small “portholes” in the endothelium to enter the peripheral circulation. Normoblasts and immature granulocytes, however, and enter the circulation in small numbers. The few normoblasts that escape into the circulation are removed by the spleen. Their presence in the peripheral blood indicates one of several possibilities:8
- The bone marrow barrier has been disrupted. The resulting release of excessive normoblasts can overwhelm the ability of a normal spleen to clear them from circulation.
- Hypoxic erythropoietin-induced compensatory erythropoiesis, as seen for example in hemolytic anemia, acute bleeding and severe hypoxic stress, results in intense marrow erythropoietic activity.
- Ineffective erythropoiesis may result in premature release of nRBCs into the peripheral blood.
- Extramedullary hematopoiesis has been activated, which lacks the normal barriers for release of nRBCs into the circulation.
- Hyposplenism or asplenia.9
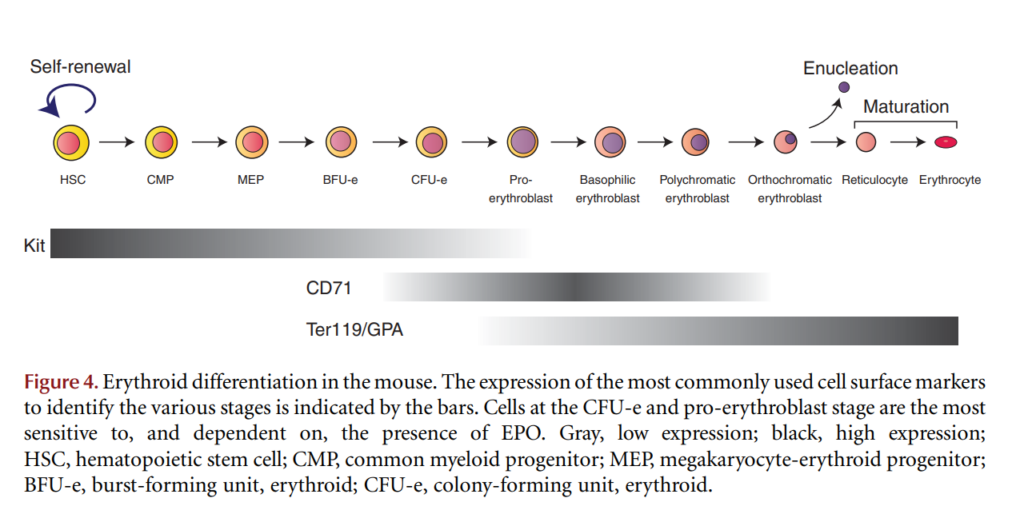
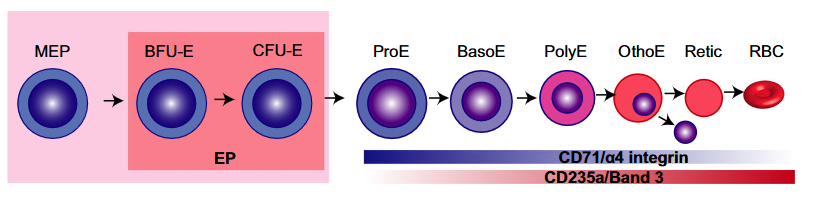
Classification
- According to maturation stage:
- Most are at the polychromatophilic or orthochromatophilic stage
- According to pathological changes:
- Normal
- Megaloblastic:
- Nuclear cytoplasmic asynchrony
- Seen in megaloblastic anemia
- Dysplastic:
- Nuclear budding
- Nuclear fragmentation
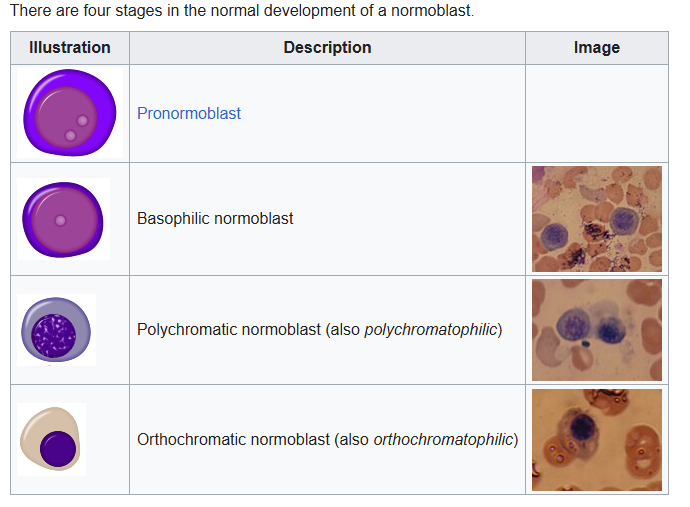
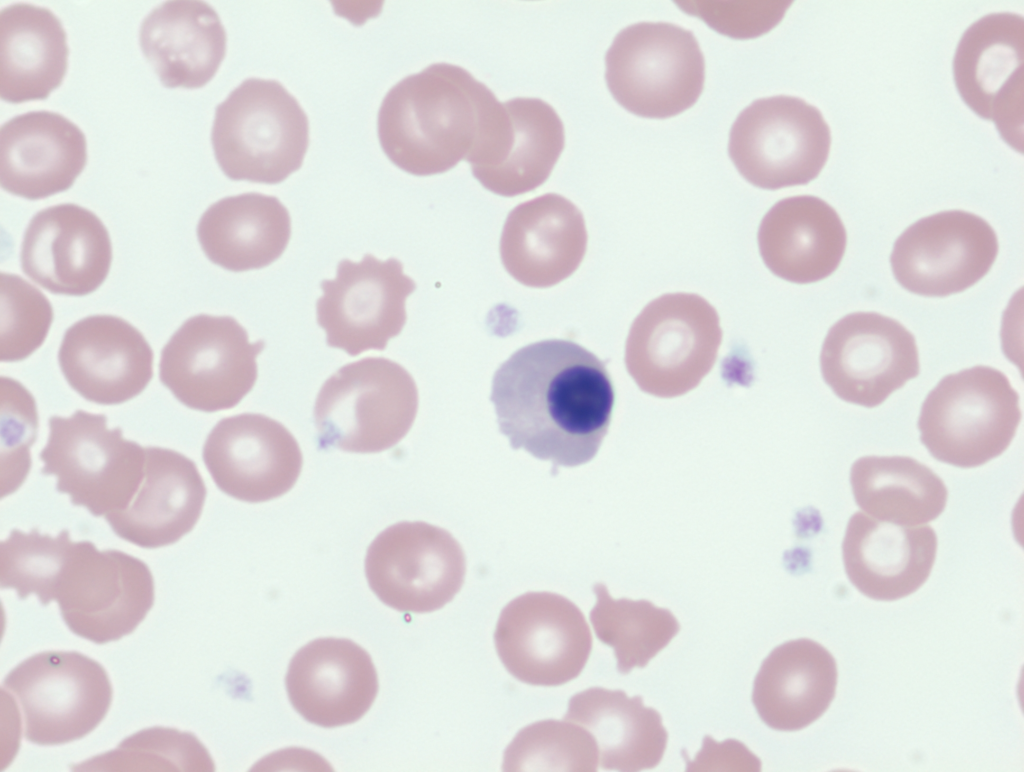
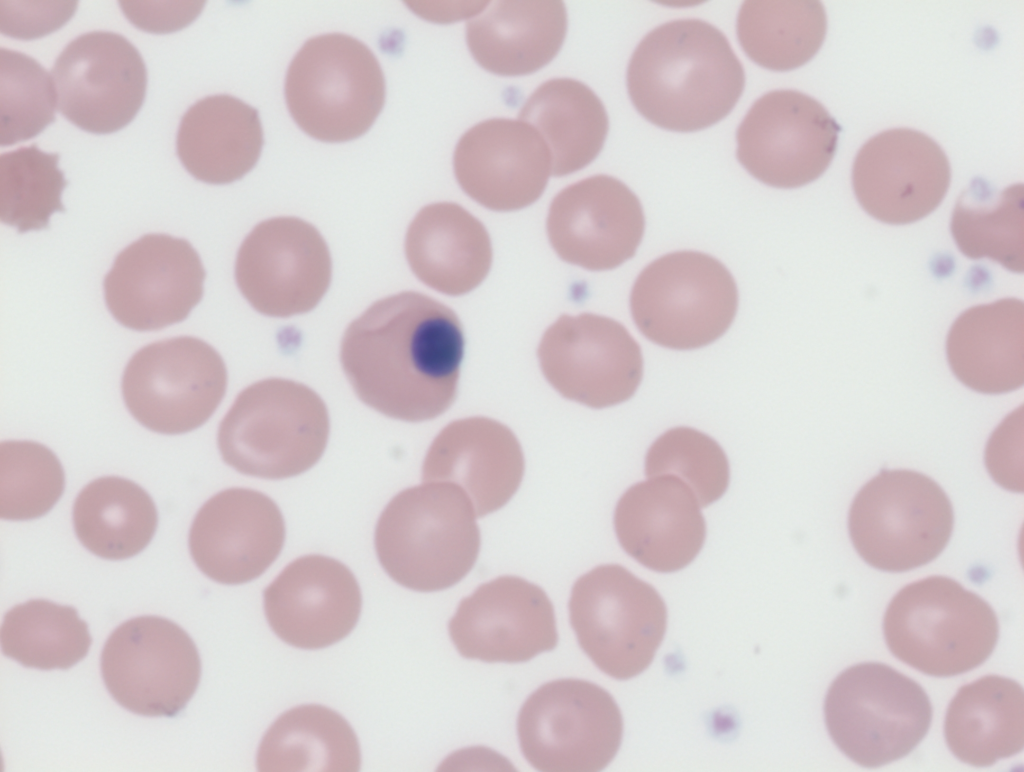
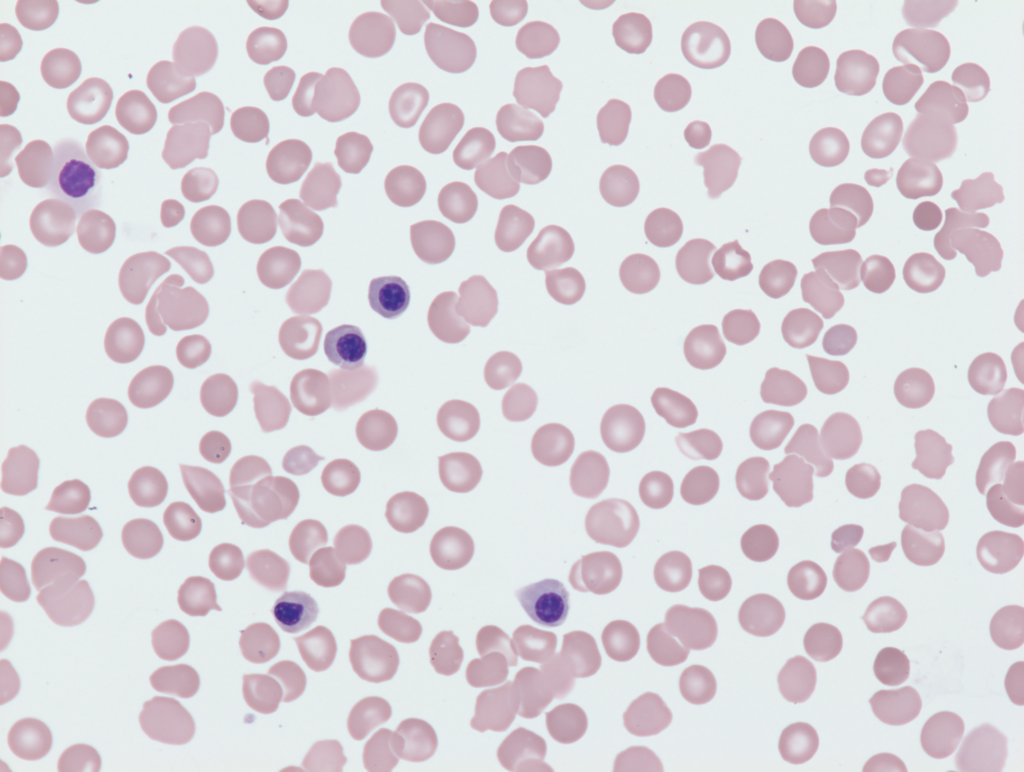
Measurement
- Manual morphometry:
- The traditional method for identification and enumeration of nRBCs is by morphometric evaluation via light microscopy (morphological eye count).
- Methods:
- Nucleated RBCs are counted as part of the overall white blood cell (WBC) count.
- 100 nucleated cells are counted on a Wright-stained peripheral blood smear by highly trained laboratory staff.
- The number of nRBCs is reported as a percentage of the nucleated cells.
- Intepretation:
- Any number of nRBCs detected by this method is considered abnormal.
- Many laboratories still rely on reference intervals (RIs) established from manual methods.
- Disadvantages of manual counting:10
- Time consuming
- Subjective
- Requires a highly trained observer.
- Using this technique, it is difficult to detect nRBC concentration of less than 100 per μL or 1 nRBC per 100 WBCs.11
- Imprecision because only 100-200 WBCs are counted (significant propensity for interobserver and intraobserver variability).
- Because nRBCs have nuclei that are similar in size and shape to lymphocytes, they are erroneously recognized and counted as WBCs, resulting in an inaccurate high WBC count and automated differential (lymphocyte) count. It is necessary to correct the leukocyte count for the nRBC count.12
- Automated counters:
- The first generations of automated hematology analyzers did not have the ability to signal the presence of NRBCs.
- Further advances in technology resulted in the ability of generating a “suspect flag”.
- More recently, automated hematology analyzers are able to count nRBCs quickly (providing an absolute count) and with a high level of accuracy and precision.
- Advantages of automated counting:
- High throughput.
- Fast turnaround time.
- Reliable.
- Excellent correlation with manual method (see graphic below).
- > 20,000 cells are counted (vs. 100 for manual count).
- Total leukocyte and lymphocytes counts are corrected for number of nRBCs.

Differential diagnosis
- Differential diagnosis:
- Physiologically seen at birth and disappear in 3-5 days.
- Outside of the neonatal period, the presence of nRBCs in the peripheral blood indicates a pathological process.
- nRBCs are seen in both hematological and non-hematological conditions.
- Their presence in the peripheral blood usually indicates increased erythroid activity, such as severe hypoxic states and hemolytic acute episodes, or damage to the bone marrow microenvironment from hematologic neoplasm, myelofibrosis, or metastatic malignant neoplasms. As mentioned above, hypo/asplenia can also lead to nRBCs.
- Recently, it has been observed that the duration and persistent presence of nRBCs in peripheral blood is linked to disease severity and more serious prognosis.
- Causes of increased release of nRBCs into peripheral blood:
- Compensatory erythropoiesis:
- Hemolytic anemia
- Hemorrhage
- Severe hypoxia
- Erythropoietin-induced erythropoiesis
- Bone marrow infiltration:
- Leukemia
- Lymphoma
- Myelofibrosis
- Multiple myeloma
- Myelodysplasia
- Solid tumor metastases
- Pernicious anemia
- Extramedullary hematopoiesis:
- Myelofibrosis
- Congenital hemolytic anemia
- Polycythemia vera
- Hypo/asplenia
- Other:
- Sepsis
- Liver disease
- Compensatory erythropoiesis:
Primary Literature
- Hwang et al, 2016
- Study Purpose and Methods:
- The study validated the automated nucleated RBC (nRBC) enumeration on the Sysmex XE-5000 hematology analyzer and assessed the frequency and clinical significance of nRBCs in a large hospital setting.
- Automated nRBC counts were compared to manual counts using 463 peripheral blood (PB) samples from patients with diverse hematologic conditions.
- Analysis of 360,504 consecutive blood samples determined the frequency of nRBCs across different patient populations.
- Key Findings:
- Correlation Between Automated and Manual Counts:
- Excellent correlation was found between automated and manual nRBC counts (Pearson’s r = 0.97 for the full range), supporting the reliability of automation.
- Correlation was weaker with lower nRBC numbers (r = 0.70 for samples with ≤20 nRBCs/100 WBCs, r = 0.47 for ≤5 nRBCs/100 WBCs), likely due to sampling error inherent in the manual 100-cell method.
- Impact of Analyzer Flags:
- The “NRBC Abn Scattergram” flag was present in 35.3% of samples with nRBCs.
- In flagged samples (n = 206), correlation for corrected WBC counts (accounting for nRBCs) remained strong (r = 0.97).
- Most flagged samples (85.4%) had ≤20 nRBCs/100 WBCs and could be automatically released without manual review, improving lab efficiency.
- Frequency of nRBCs in Different Patient Groups:
- Highest nRBC rates were seen in abnormal CBC morphology groups: 26.0% (CBC only) and 51.8% (CBC+DIFF).
- Intermediate frequency in samples with normal morphology but at least one abnormal parameter: 4.0% (CBC), 7.8% (CBC+DIFF).
- Low-level nRBCs (0.2–1.3/100 WBCs) appeared in 0.5% of samples with normal morphology and CBC parameters, commonly in patients without evidence of increased erythropoiesis or marrow pathology.
- Most samples containing low-level nRBCs and normal CBCs were from individuals with mild or unrelated conditions.
- Clinical and Laboratory Implications:
- Automated nRBC measurement enables rapid, accurate, and high-throughput detection, minimizing the need for manual smear reviews.
- The technology can identify a subset of patients with low-level nRBCs (under 1.5/100 WBCs) and otherwise normal CBCs—a finding that may be physiologic rather than pathologic in many cases.
- Automating nRBC assessment reduced manual workload, with over 99% of specimens not requiring manual nRBC enumeration.
- Conclusion:
- The Sysmex XE-5000 provides accurate, efficient nRBC counts, correlating strongly with manual methods except at very low levels, likely due to limitations of manual counting.
- Low-level nRBCs detected by automated analyzers in patients with normal CBCs often are not associated with disease, suggesting the possibility of redefining the clinical significance of such findings in contemporary laboratory practice.
- Correlation Between Automated and Manual Counts:
- Study Purpose and Methods:
- Pikora et al, 2023
- What Are NRBCs?
- NRBCs are immature erythrocyte precursors, normally found in bone marrow but rarely in the circulation of healthy adults.
- In fetuses and neonates, NRBCs may circulate, but they usually disappear within the first month of life.
- Clinical Value of NRBC Measurement:
- NRBC count is a low-cost, simple laboratory test, though seldom utilized in routine practice.
- NRBC presence in peripheral blood generally indicates strongly stimulated erythropoiesis (e.g., hypoxia, blood loss, hemolysis) or disruption of the blood–bone marrow barrier.
- Prognostic and Diagnostic Utility in Specific Populations:
- Newborns and Neonates:
- Hypoxia: Elevated NRBCs at birth suggest intrauterine hypoxia, with NRBCs appearing 24–36 hours after hypoxic insult and correlating with both severity and duration.
- Asphyxia: Higher NRBC counts align with increased risk of hypoxic-ischemic encephalopathy (HIE) and its severity; combining NRBC counts with EEG improves prognostic value.
- Mortality and Morbidity: Higher NRBC counts in the first days predict greater risk of unfavorable outcomes and mortality in very low birth weight infants, and correlate with complications like ROP and NEC.
- Pediatrics and Adults:
- Critical Illness: In children and adults, NRBCs consistently predict poor prognosis and increased mortality in ICU patients, including those with trauma, sepsis, ARDS, and acute pancreatitis. A positive NRBC test is often an independent marker of higher risk.
- Cardiac Conditions: In cardiac ICUs, NRBCs add predictive power beyond established scoring systems (e.g., APACHE II).
- Hematologic Disorders:
- Chronic Myeloid Leukemia (CML): Persistent NRBCs can indicate failure of molecular remission during imatinib therapy.
- Myelodysplastic Syndromes (MDS): Impaired mitophagy in NRBCs is linked to anemia severity in MDS.
- Thalassemia Syndromes: NRBC counts reflect ineffective erythropoiesis, particularly in beta thalassemia major, and may help guide transfusion strategies; they also show potential for non-invasive prenatal diagnosis when fetal cells are isolated from maternal blood.
- Conclusion:
- NRBC count is a simple, cost-effective, and widely available laboratory marker with strong potential as a diagnostic and prognostic tool in:
- Neonatal medicine (hypoxia, asphyxia, mortality risk)
- Critical care (ICU, sepsis, ARDS, cardiac events, acute pancreatitis)
- Selected hematological disorders (CML, MDS, thalassemia)
- Elevated NRBCs in peripheral blood should be viewed as a possible indicator of severe disease or poor prognosis, prompting increased monitoring and intervention. However, its integration into regular clinical workflows awaits further large-scale validation.
- NRBC count is a simple, cost-effective, and widely available laboratory marker with strong potential as a diagnostic and prognostic tool in:
- Newborns and Neonates:
- What Are NRBCs?
- Meredith et al, 2024
- Background:
- Nucleated red blood cells (nRBCs) are typically absent from adult and child peripheral blood, with their presence traditionally seen as pathological. Neonates may have nRBCs, but these diminish by around one month of age. Older reference intervals (RIs) for adults, largely based on manual microscopy, flagged any detectable nRBC as abnormal. With the advent of more sensitive automated analyzers, these reference intervals may no longer be appropriate.
- Study Design & Methods
- Study Setting: University-based outpatient clinical lab, using the Sysmex XN-10 analyzer.
- Population: 405,300 CBCs reviewed; after strict criteria to ensure samples were from healthy, non-hospitalized individuals without confounding pathology (like MS or oncology orders), 66,498 samples remained.
- Inclusion Criteria: Normal CBC values (hemoglobin, MCV, platelets, RBC, RDW, WBC), excluding conditions known to spuriously elevate nRBCs.
- Evaluations: Compared nRBC counts across age and sex, calculated means, medians, SDs, and proportion with counts above prior RI upper limit.
- Results:
- Previous Reference Interval (RI): 0.00 to 0.01 × 10⁶/µL.
- Findings:
- 338 of 66,498 (0.5%) exceeded the old upper RI limit; 336 just slightly above, only two >0.10 × 10⁶/µL.
- Over 99% of healthy individuals had nRBC ≤0.10 × 10⁶/µL.
- Mean nRBC values were very low in all age and sex groups; medians across all subgroups were 0.
- Females had slightly lower counts than males (by 0.0002 × 10⁶/µL).
- Implications & Recommendations:
- New Reference Interval: Proposed upper limit: 0.10 × 10⁶/µL for healthy adults and children after the neonatal period.
- Clinical Impact: Raises the threshold for an “abnormal” result, cuts unnecessary referrals and workups, reduces healthcare costs, and addresses patient anxiety due to over-sensitive reporting.
- Analyzer Sensitivity: Automated analyzers (like the Sysmex XN-10) detect very low levels missed by manual methods—most of these are likely not clinically significant in otherwise healthy individuals.
- Other Findings: High altitude residency (5280 ft in Denver) could potentially affect results, although not definitively determined.
- Conclusion:
- Low concentrations of nRBCs (up to 0.10 × 10⁶/µL) are detectable in a small percentage of healthy individuals using modern analyzers. Raising the reference interval aligns reporting with clinical reality and reduces unnecessary interventions, better reflecting contemporary hematology practice. This updated RI is strongly supported by robust outpatient data and has already reduced low-value care and referrals in the authors’ institution.
- Low concentrations of nRBCs (up to 0.10 × 10⁶/µL) are detectable in a small percentage of healthy individuals using modern analyzers. Raising the reference interval aligns reporting with clinical reality and reduces unnecessary interventions, better reflecting contemporary hematology practice. This updated RI is strongly supported by robust outpatient data and has already reduced low-value care and referrals in the authors’ institution.
- Background:
Why did mammalian RBCs lose their nucleus?
Several hypotheses:
- Maximizing Hemoglobin Packing:
- Removing the nucleus frees up intracellular space, allowing mammalian RBCs to carry more hemoglobin per cell.
- This increases the oxygen-carrying capacity of the blood without increasing cell size (which would slow capillary transit).
- Flexibility and Microcirculation:
- Enucleated RBCs can adopt their classic biconcave disk shape, making them highly deformable.
- This flexibility is critical for squeezing through very narrow capillaries (sometimes narrower than the cell itself).
- Nucleated RBCs, being stiffer and less deformable, would be less efficient in dense capillary networks.
- Reduced intrinsic viscosity:
- Anucleate cells have less intrinsic viscosity → they make the blood flow more efficiently for a given hematocrit.
- Metabolic Efficiency:
- Without nuclei and organelles, RBCs avoid using oxygen themselves (no mitochondria).
- They rely on anaerobic glycolysis, conserving the oxygen they transport for tissues.
- Enucleation thus reduces “oxygen consumption overhead.”
- Immune/Pathogen Defense Hypotheses:
- Some suggest enucleation reduced susceptibility to certain intracellular parasites or viruses that exploit nuclei (though malaria still infects mammalian RBCs).
- This is more speculative but may have offered selective advantage in certain ecological niches.
Evolutionary Trade-off
- Lost nucleus → no repair/protein synthesis, shortened lifespan (120 days max in humans).
- Gained → lower viscosity + higher oxygen transport capacity → enormous evolutionary advantage for endotherms.
Prognosis
- Elevated levels of nRBCs have been shown to predict mortality in:
