I tweeted a poll 4/9/24 asking how you would treat a patient who had evidence (based on labs and image I showed) of purpura fulminans.
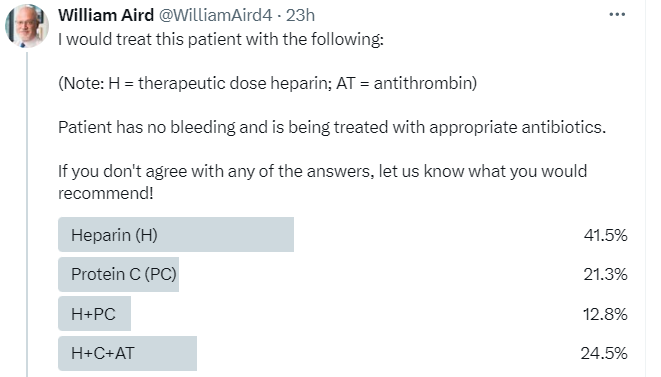
The most popular answer was heparin, though many respondents chose protein C (PC) or a combination of heparin/antithrombin (AT) and PC. In fact, there is no “right answer”. Purpura fulminans (PF) is a rare disorder, and there are no randomized control trials exploring the benefit of PC concentrate, AT concentrate or heparin in this condition. Since PF is characterized by disproportionate reduction in circulating levels of PC, some authors favor using PC concentrates, while others argue that a marked reduction in endothelial expression of thrombomodulin in PF would negate activation of exogenously administered PC. Because PF is a thrombotic subtype of DIC, some authors as well as the British Society of Haematology guidelines support the use of therapeutic doses of heparin in this situation. There is no evidence that AT concentrate is beneficial in patients with PF, and few authors advocate for its use.
Case
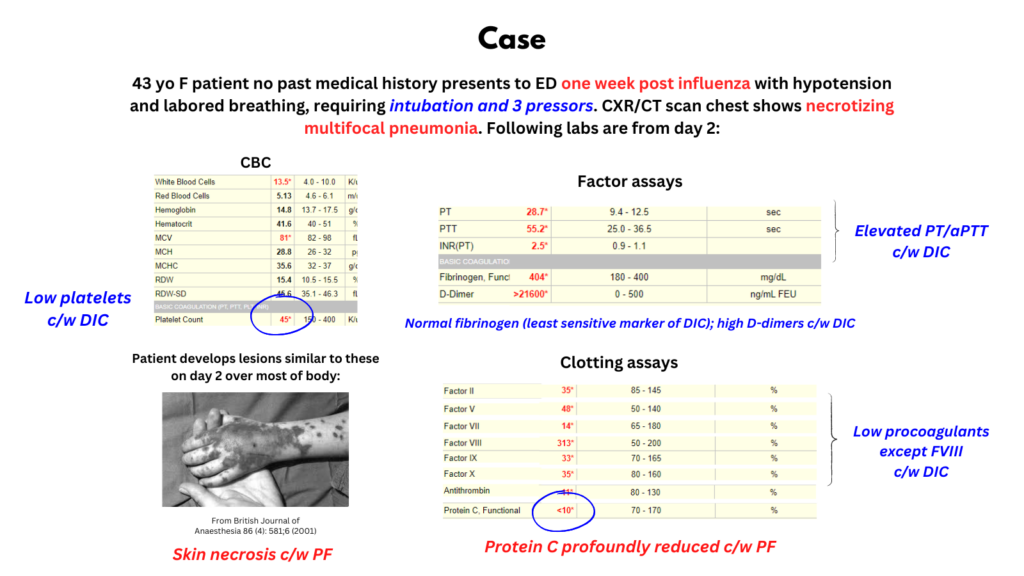
- 43 yo F previously well presented to ED one week after influenza with shortness of breath and hypotension. He was intubated and required 3 pressors for blood pressure support:
- CXR and CT chest showed necrotizing multifocal pneumonia. She received vancomycin, cefepime, azithromycin, meropenem, clindamycin and tobramycin and was treated with three pressors.
- Within hours, she developed purpuric lesions over most of his body.
- Blood cultures grew Group A streptococcus, and antibiotics were changed to linezolid and zosyn.
- Her labs showed:
- Thrombocytopenia on admission, which progressed during hospitalization (105 -> 27)
- Leukocytosis on day 2, which also progressed over time
- Progressive coagulopathy with increased PT and aPTT
- Initial fibrinogen of 700, which decreased to 256 by day 3
- Factor assays on day 2:
- FII 35%
- FV 48%
- FVII 14%
- FVIII 313%
- FIX 33%
- FX 35%
- AT 11%
- PC <10%
- PS 67%
- Elevated creatinine and LFTs on day 2
- ID diagnosis: “Overall, suspect that he developed a post-influenza necrotizing PNA with Group A streptococcus.”
- Hematology diagnosis: post-infectious purpura fulminans. Treatment recommendations were the following:

- The patient had a fulminant course with multiorgan failure and died on day 4.
Definition
- Purpura fulminans (PF) is a rare, life-threatening syndrome characterized by disseminated intravascular coagulation (DIC) and typically resulting from acquired protein C deficiency leading to extensive endovascular thrombosis of medium-sized vessels resulting in hemorrhagic infarction and skin necrosis (cutaneous purpura) and multiorgan failure with an extremely high mortality rate.1
- Disseminated intravascular coagulation (DIC) – The Scientific and Standardization Committee (SSC) on DIC of the International Society on Thrombosis and Haemostasis (ISTH) defines DIC as “an acquired syndrome characterized by the intravascular activation of coagulation with a loss of localization arising from different causes; can originate from and cause damage to the microvasculature, which if sufficiently severe, can produce organ dysfunction.”
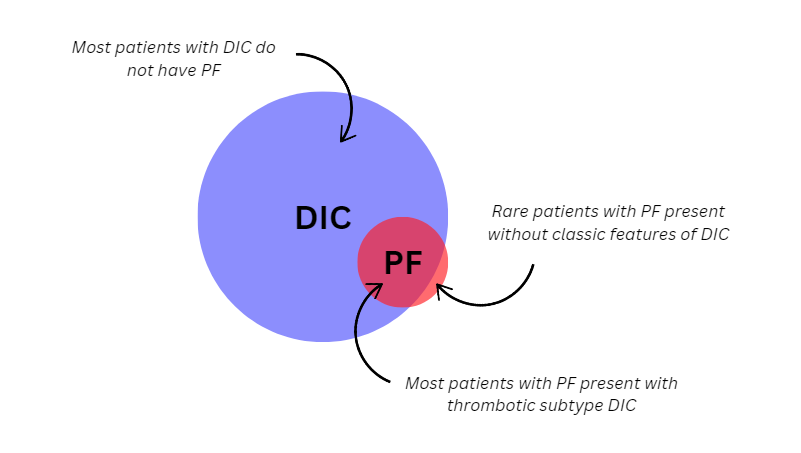
- PF is a highly thrombotic subtype of DIC, though it may rarely occur without classic changes of DIC. Patients without DIC features (thrombocytopenia, elevated PT/D-dimers, low fibrinogen) may present with skin necrosis and low circulating levels of protein C alone.2
History
- The syndrome of PF was first described by Guelliot in 1884.3
- The name was first applied by Henoch in 1887.4
Epidemiology
- Purpura fulminans occurs most often in children with a bimodal peak in incidence:5
- 1 to 3 years old
- 16 to 18 years old
- The peak in adolescence is explained in part by the higher incidence of infection with N. meningitidis in this age group.6
- Accounts for <0.5% of cases of septic shock and <1% of patients with disseminated intravascular coagulation.7
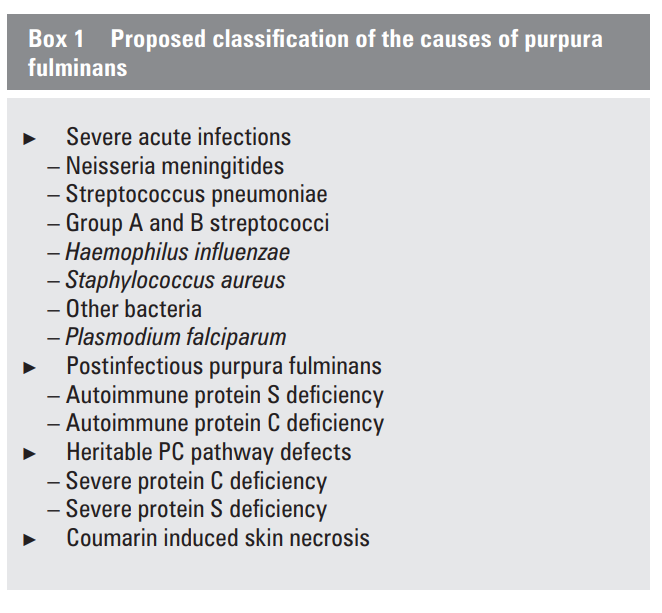
Causes
- Sepsis (acute infectious PF):8
- Neisseria meningitidis:
- The most common infectious cause of PF.
- 15%-25% of patients with meningococcal infection develop PF.
- Mortality of meningococcal infection complicated by PF is 20%-60% compared to ~15% in uncomplicated cases.
- Patients with congenital or acquired complement deficiency following administration of complement inhibitors such as eculizumab have an increased risk of developing meningococcal PF.9
- Streptococcus pneumoniae:
- Haemophilus influenzae:1314
- Capnocytophaga canimorsus1516 –
- Staphylococcus aureus17
- Streptococcus pyogenes18
- Endotoxin-producing gram-negative bacteria
- Varicella (the most common viral trigger)
- Neisseria meningitidis:
- Inherited deficiency:19
- Neonates with severe inherited homozygous deficiencies of protein C (and rarely protein S).
- Skin necrosis with subsequent gangrene typically develops on the legs and male genitalia within a few hours or days of birth.
- Cerebral venous thrombosis may occur, resulting in neurologic symptoms.
- Treatment with protein C concentrate can be life saving and prevent irreversible end-organ damage.
- Post-Infectious:20
- Post-infectious autoimmune disorder, occurring 7 to 10 days following infection – most often Varicella or Streptococcus – believed to occur secondary to acquired autoantibodies against protein C or protein S.
- Cross-reacting IgG autoantibodies that increase clearance of protein S from the circulation are thought to trigger post-infectious PF and patients tend to be lupus anticoagulant positive at the time of presentation.
- Lesions tend to occur in the thighs, lower legs, and buttocks as well as the scrotum and penis in men.
- There is usually distal extremity sparing without circulatory collapse, and this etiology is associated with a lower mortality rate (~15%).
- For patients who survive the acute phase of illness, many experience spontaneous resolution of the autoantibodies by three months.
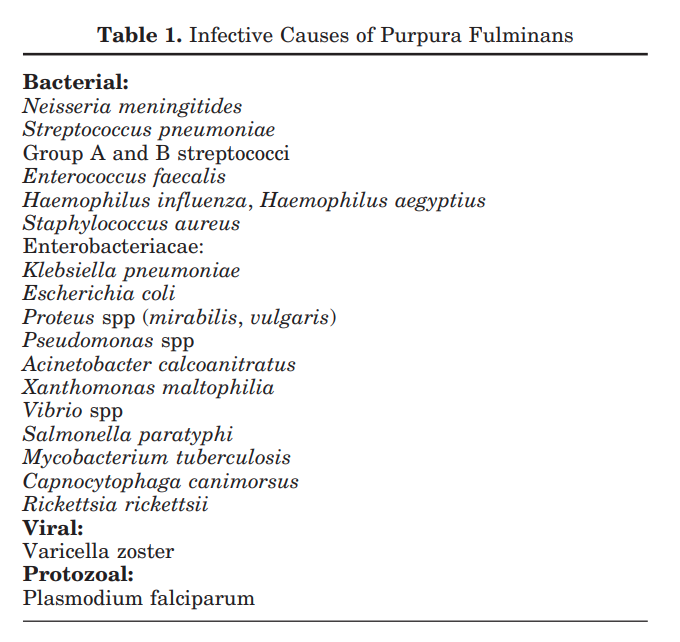
Pathophysiology
- Results from dysfunction of the body’s natural anticoagulant mechanisms, particularly abnormalities in the function of protein C (PC) and associated proteins.
- Normal functions of protein C :
- PC is a vitamin K-dependent serine protease that inhibits coagulation and has anti-inflammatory and cryoprotective properties:21
- Protein C in coagulation:
- In response to a prothrombotic stimulus/thrombus formation, PC binds to the endothelial PC receptor (EPCR), forming a complex with thrombomodulin (TM)-bound thrombin, which then activates PC by cleavage at Arg 506.
- Once activated, PC cleaves and inactivates the cofactors FVa and FVIIIa as well as plasminogen-activator inhibitor 1 (PAI-1), leading to inhibition of coagulation and lysis of preformed clot.
- Protein C in cytoprotection:
- In addition to its role in regulating coagulation, PC plays a protective role in endothelial cell signaling.
- Activated PC (APC) in the presence of EPCR cleaves protease-activated receptor-1 (PAR-1), which then triggers a signaling cascade that activates anti-inflammatory and anti-apoptotic genetic programs and promotes endothelial barrier function.
- Animal models suggest that APC-mediated cytoprotection plays an important role in host response to infection.
- Protein C in coagulation:
- PC is a vitamin K-dependent serine protease that inhibits coagulation and has anti-inflammatory and cryoprotective properties:21
- Dysfunctional TM/APC system in purpura fulminans:
- Pathophysiology of PF includes bacteria-induced alterations in hemostatic balance and vascular integrity primarily at the level of TM/APC:22
- There is depletion of circulating anticoagulants protein C, protein S and antithrombin.
- Dysfunction in the endothelial TM/PC system also occurs:
- Animal models show tight interaction between Neisseria meningitidis and endothelial cells, triggering subsequent vascular damage.
- Bacteria adhere to the endothelium and proliferate in shear stress-resistant aggregates that eventually fill the vascular lumen through a filamentous adhesive structure called type IV pilus.
- Type IV pili have been demonstrated as essential virulence factors for the progression of meningococcemia to PF.23
- Skin biopsies of pediatric patients with PF secondary to meningococcal sepsis showed severely decreased expression of endothelial thrombomodulin and EPCR associated with increased plasma levels of soluble TM in PF patients compared to unaffected controls, leading to conclusion that:
- An unknown property of the infecting organism led to cleavage and loss of endothelial TM with a subsequent inability to efficiently activate PC.
- This results in unopposed activation of the coagulation system and overwhelming thrombosis.
- Per Bendapudi et al: “The initiating event in PF is hypothesized to be loss of TM from the endothelial surface in response to infection. As a result, conversion of PC to APC is impaired, and coagulation proceeds unchecked.”
- Pathophysiology of PF includes bacteria-induced alterations in hemostatic balance and vascular integrity primarily at the level of TM/APC:22
- Complement activation:
- One recent study using whole-exome germ line sequencing showed that patients with PF have complement system variants in both soluble complement plasma proteins and complement receptors (CRs),24 suggesting a likely genetic link between maladaptive hyperinflammation in purpura fulminans and the complement system.
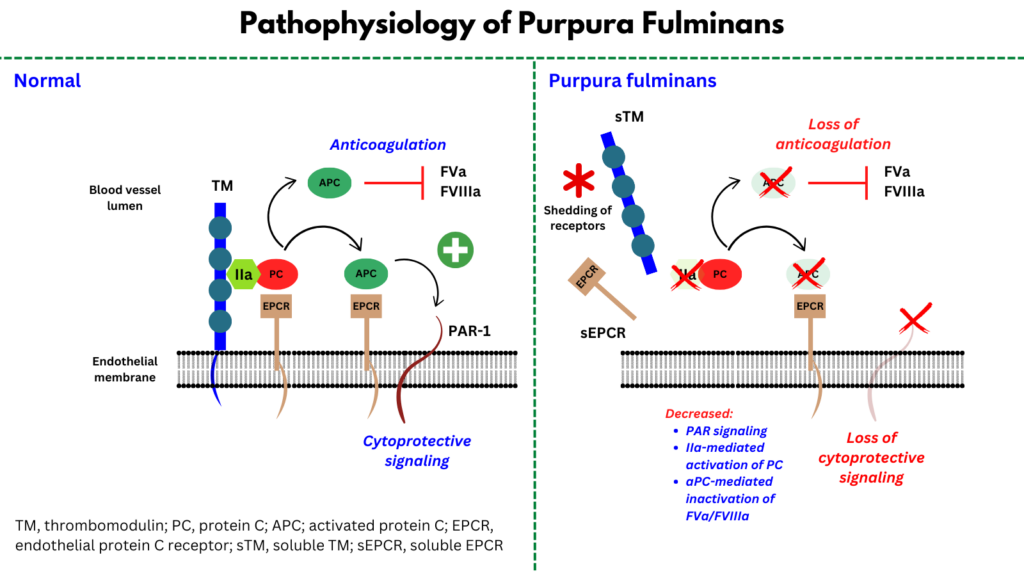
Clinical presentation
- Purpura fulminans often affects young patients with no previous comorbidities.25
- Prodrome prior to development of rash includes non-specific symptoms such as:26
- Fever
- GI symptoms
- Headache
- Lower limb pain
- Myalgia/arthralgia
- Purpuric rash:27
- Predominant nonacral skin necrosis.
- Typically begins within hours of admission to the intensive care unit (ICU) for acute circulatory failure.
- Begins as erythematous macules (petechiae coalesce to form purple ecchymoses) on the trunk and extremities, which rapidly become indurated, nonblanching with thin, irregular, advancing borders.28
- Central areas of necrosis then develop and bullae may form if hemorrhage into the necrotic dermis occurs.
- The hallmark of hemorrhagic infarction is the development of hemorrhagic bullae, which can progress to frank skin necrosis and gangrene.29
- Within 24 to 48 hours, this pattern progresses to irreversible full-thickness necrosis of the skin.
- The initial clinical findings correlate histologically with microthrombi of small dermal vessels.
- Most patients present with septic shock.30
- It is not unusual for death to occur from end organ damage secondary to thrombosis of small and medium-sized vessels many days or even 1 to 2 weeks after successful clearance of the responsible organism.31
Diagnosis
- Clinical diagnosis that requires a high index of suspicion early in the patient’s presentation.
- Microbiological documentation is obtained in more than 90% of cases.32
- Skin biopsy may show:33
- Occlusion of dermal venules and capillaries by microthrombi, resulting in hemorrhagic infarction.
- Swelling of endothelial cells
- Capillary dilation with congestion of the vessels by erythrocytes
- Minimal to mild inflammatory neutrophil infiltrate may be found in the perivascular region
- Coagulative necrosis of the dermal and subcutaneous tissues with extensive dermal hemorrhage manifested as gangrenous necrosis.
- Laboratory parameters consistent with DIC, including:
- Prolonged coagulation times
- Decreased fibrinogen
- Elevated d-dimers
- Thrombocytopenia
- Reduced levels of:
- Protein C
- Protein S
- Antithrombin
- Elevated C-reactive protein (CRP) in the setting of an inappropriately low erythrocyte sedimentation rate (ESR).34
Differential diagnosis
- Warfarin-induced skin necrosis
- Cryoglobulinemic vasculitis
- Catastrophic anti-phospholipid syndrome
- Heparin-induced thrombocytopenia
- Henoch-Schonlein purpura
Treatment
- There are no large clinical trials of patients with purpura fulminans. Treatment recommendations are based on expert opinion.
- Prompt administration of a parenteral β-lactam antibiotics as soon as the diagnosis is suspected.
- Supportive care for organ failure.
- Protein C concentrate:
- Colling and Bendapudi:
- It has been argued that patients with PF might benefit from treatment with protein C (PC).
- The goal of treatment with PC is to rapidly replete PC and provide substrate to the remaining pool of endothelial thrombomodulin (TM).
- Based on several single-arm trials, it appears that treatment with PC can normalize PC levels and markers of DIC and possibly reduce morbidity and mortality.35
- “Nevertheless, the logic of PC repletion in PF appears sound, and this approach warrants further investigation through well designed trials.”
- Bendapudi et al:
- “Although therapy with PC concentrate has been proposed as a strategy to target the underlying pathophysiologic lesion in PF, the belief that endothelial TM loss is an early event in PF that renders infused PC ineffective has limited its widespread adoption.”
- The authors show that TM protein can persist at the endothelial surface for up to 72 hours after initial presentation in some patients before declining to an undetectable level. They state “This observation is consistent with previous work demonstrating that infused PC is rapidly converted to APC in PF patients and suggests that supplemental PC can be effective, even after patients present with apparent failure of the TM-PC pathway.”
- “Although results from this single case report should be interpreted with caution, our data support the idea that, during the hyperacute phase of PF, endothelial TM remains intact, and PC is consumed extremely rapidly (a process that requires the presence of functional TM). Endothelial TM loss occurs later and is a secondary event. Therefore, it is overwhelming thrombin generation and the resultant depletion of PC, not the absence of endothelial TM, that is the primary driver of microvascular thrombosis in PF. We hypothesize that PC supplementation, together with other anticoagulant therapies, served to mitigate the effects of prothrombotic stimuli from the microorganism as antibiotics gradually cleared the infection.”
- Colling and Bendapudi:
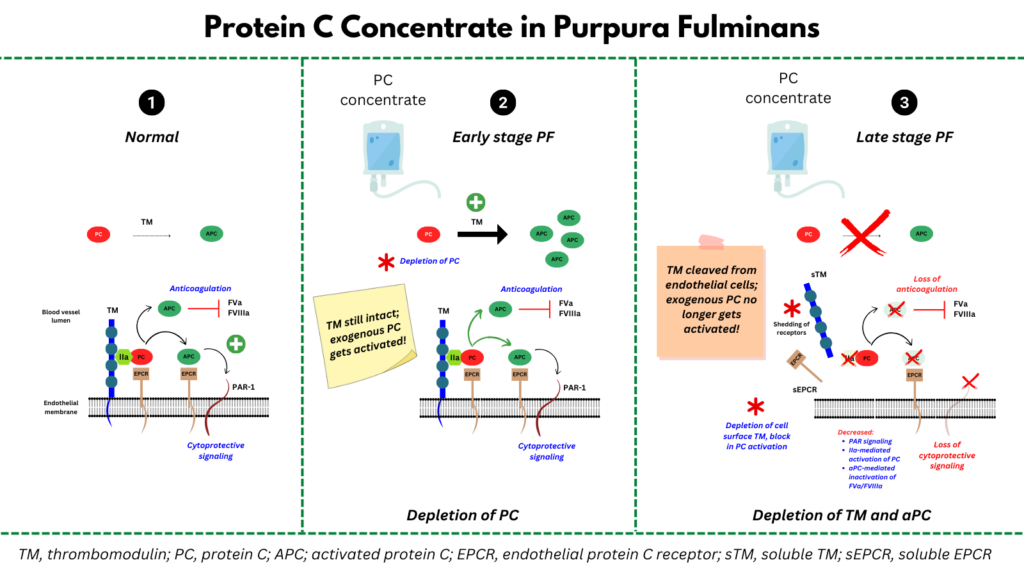
- Protein C concentrate (cont’d):
- Warkentin:
- “Some experts advise early protein C replacement therapy in patients with severe meningococcemia. However, in order to become activated, protein C requires the presence of thrombomodulin on the surface of endothelial cells. Since injured endothelial cells down-regulate and shed thrombomodulin, current experimental approaches include the infusion of recombinant human soluble thrombomodulin.
- Up-to-Date:
- “Patients with purpura fulminans, including adults, appear to benefit from the administration of protein C concentrate. In one series of 12 patients with purpura fulminans so treated, none died despite a predicted mortality rate of 60 to 80 percent. Intravenous dosing is 100 IU/kg as an initial bolus followed by 50 IU/kg every six hours until D-dimer normalizes or shows a decreasing trend.
- The administration of fresh frozen plasma as a source of protein C is more difficult because of the short half-life of protein C in the plasma. Two to three units of FFP may be administered approximately every six hours if tolerated.
- In contrast, protein C deficiency without purpura fulminans is not an indication for using protein C concentrate.
- Primary data:
- Retrospective study of 12 patients with Neisseria meningitidis and PF:
- Age 3 months and 27 years old.
- Protein-C concentrate was started within 18 h of admission in ten of the 12 patients.
- All patients were treated with antibiotics, fluid resuscitation (albumin 4·5%), inotropic drugs, and mechanical ventilation.
- Hemodiafiltration began within 12 h of admission in eight patients.
- The mean protein-C concentration was 0·20 IU/mL [SD 0·05].
- Antithrombin concentrations were below the normal range in 11 of the 12 patients (mean 0·56 IU/mL [0·17]) but always higher than the individual’s protein-C concentration.
- A test dose (10 IU/kg) of purified protein-C concentrate (125 IU/mL) was given intravenously over 10 min. A loading dose (100 IU/kg) and then a continuous infusion (15 IU/kg/h ) of protein-C concentrate were given and the dose was adjusted daily to keep the plasma concentration between 0·8 IU/mL and 1·2 IU/mL.
- Patients were anticoagulated with intravenous unfractionated heparin (10–15 IU kg/h), to achieve activated partial-thromboplastin-time ratios between 1·5 and 2·0.
- Antithrombin III concentrate was used in one patient who had an antithrombin concentration of 29 IU/mL because heparin is effective only if antithrombin concentrations are above 35 IU/mL. The total dose of antithrombin concentrate given was 150 IU/kg units.
- The mean duration of therapy:
- Protein C 5·7 (range 4·0–8·0) days
- Heparin 8·1 (4·0–20·0) days
- Hemodiafiltration 4·7 (0·0–20·0) days
- Mechanical ventilation 8·5 (range 4·0–17·0) days
- Inotropic support 4 (3–7) days
- Two patients had lower-limb amputations; both of the patients who had amputations had received protein-C concentrate and heparin later than the other patients (60 h [SD 19·77] vs 12 h [3·13]).
- One patient had fasciotomies (patient 6) in both legs and right arm.
- One patient developed chronic renal failure despite early use of protein-C concentrate.
- All patients survived.
- No adverse reactions to the protein-C concentrate or antithrombin concentrates were seen.
- None of the patients treated with heparin had a significant hemorrhage.
- Retrospective study of 12 patients with Neisseria meningitidis and PF:
- A note about activated protein C:
- Early studies showed promising results in patients with severe sepsis (PROWESS study).
- The benefit in morality was not confirmed in subsequent trials including a large population of patients with septic shock (PROWESS-SHOCK trial), leading to withdrawal of activated protein C from the market.
- Activated protein C has never been tested in a cohort of patients with PF. In theory, it might be more efficacious than protein C concentrates because of the reduction of thrombomodulin on the surface of endothelial cells resulting in reduced thrombin-mediated activation of exogenously administered PC. In other words, it bypasses the need for endogenous activation.
- However, activated protein C is no longer available for use.
- Warkentin:
- Heparin:
- Colling and Bendapudi:
- Older trials of heparin, primarily in pediatric patients with severe meningococcal infection did not demonstrate a survival benefit, but these studies were limited by:
- Small sample sizes
- Non-standardized inclusion criteria
- Delays in initiation of heparin
- Variable heparin dosing regimens
- Limited monitoring of heparin levels
- “Randomized, double-blind, placebo controlled HETRASE study (see below) looking at the use of systemic heparin in patients with septic shock did not reproduce these results, finding no difference in organ dysfunction, length of hospital stay, or all cause mortality with use of heparin. However, only 10% of patients in each group had septic shock and there was no significant difference between the median activated partial thromboplastin time (aPTT) between the treatment and control groups, calling into question the efficacy of the study intervention.”
- “A recent meta-analysis of patients with sepsis, septic shock, and infection-associated DIC (see below) found that treatment with heparin as compared to placebo or usual care may reduce the relative rate of death by 12%.”
- “Although the true effect of heparin on outcomes remains unknown, guidelines for the diagnosis and management of DIC recommend therapeutic doses of heparin in cases of DIC where thrombosis predominates (including PF).”
- “It is important to note that prolonged baseline coagulation assay results in these patients is not a contraindication to heparin therapy, since these derangements are a marker of thrombosis, not bleeding tendency.”
- “Furthermore, the overwhelming inflammation present in PF can render these patients relatively resistant to heparin therapy due to circulating acute phase proteins that bind and inactivate heparin, e.g. vitronectin. Therefore, heparin levels must be monitored closely with the anti-Xa assay and aggressive dose increases used in the event of sub-therapeutic results.”
- Older trials of heparin, primarily in pediatric patients with severe meningococcal infection did not demonstrate a survival benefit, but these studies were limited by:
- Levi and Scully:
- Therapeutic doses of heparin are indicated in patients with clinically overt thromboembolism and may be considered in cases of extensive thrombotic manifestations, such as in PF or acral ischemia
- In flowchart for therapeutic management of DIC:
- If overt thrombo-embolism and/or organ failure related to clot formation (e.g. PF):
- Therapeutic anticoagulant treatment (e.g. unfractionated heparin IV)
- (Restoration of natural anticoagulant pathways under evaluation in clinical studies)
- If overt thrombo-embolism and/or organ failure related to clot formation (e.g. PF):
- Warkentin:
- “Whether there is any potential benefit for the use of heparin in the prevention of microthrombosis and ischemic limb injury is unknown.”
- UpToDate:
- Thrombosis appears to be more common (although still rare overall) with certain infectious causes of DIC such as severe malaria or dengue virus infection. In those cases, thrombosis can be life- or limb-threatening. Digital gangrene of the fingers or toes has been reported. Treatment with heparin in such cases is appropriate, although there are no major trials addressing the efficacy or administration of anticoagulants in this setting.
- Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology:
- In cases of DIC where thrombosis predominates, such as arterial or venous thromboembolism, severe purpura fulminans associated with acral ischemia or vascular skin infarction, therapeutic doses of heparin should be considered.
- Systematic review and metaanalysis on the efficacy and safety of heparin in patients with sepsis:
- 9 trials enrolling 2,637 patients were included.
- Eight trials were of unclear risk of bias and one was classified as having low risk of bias.
- In trials comparing heparin to placebo or usual care, the risk ratio for death associated with heparin was 0.88 (95% CI, 0.77–1.00; 2,477 patients; six trials; moderate strength of evidence).
- In trials comparing heparin to placebo or usual care, major hemorrhage was not statistically significantly increased (risk ratio, 0.79; 95% CI, 0.53–1.17; 2,392 patients; three trials).
- Conclusions: Heparin in patients with sepsis, septic shock, and disseminated intravascular coagulation associated with infection may be associated with decreased mortality; however, the overall impact remains uncertain. Safety outcomes have been underreported and require further study. Increased major bleeding with heparin administration cannot be excluded. Large rigorous randomized trials are needed to evaluate more carefully the efficacy and safety of heparin in patients with sepsis, severe sepsis, and septic shock.
- Primary data:
- Randomized, double-masked, placebo-controlled, single-center clinical trial, testing low dose continuous infusion of unfractionated heparin (UFH) as complementary treatment for sepsis:
- Three hundred nineteen patients randomly assigned to receive placebo or UFH (500 units/hour for 7 days).36
- The median length of stay in patients discharged alive (p = 0.976):
- Placebo group was 12.5 days (interquartile range = 8-20)
- Heparin group 12 days (interquartile range = 8-19.5)
- The MOD score improved equally in the two treatments arms with an average decline of 0.13 and 0.11 per day for the placebo and heparin groups (p = 0.240), respectively.
- The overall 28-day mortality (p = 0.652):
- Placebo group 16%
- Heparin group 14%
- Subgroup analyses did not show any statistically significant reduction in 28-day mortality with UFH.
- there was only one serious adverse event on a patient who received heparin but it was fully resolved without complications.
- The median length of stay in patients discharged alive (p = 0.976):
- Conclusions: Our findings suggested that UFH may be a feasible and safe intervention in sepsis. However, this study was not able to demonstrate a beneficial effect on the chosen primary outcomes or in the 28-day mortality rate.
- Three hundred nineteen patients randomly assigned to receive placebo or UFH (500 units/hour for 7 days).36
- Randomized, double-masked, placebo-controlled, single-center clinical trial, testing low dose continuous infusion of unfractionated heparin (UFH) as complementary treatment for sepsis:
- Colling and Bendapudi:
- Plasma Exchange:
- Scattered reports of its use in patients with PF.37
- PF not mentioned as an indication in the 2023 Guidelines on the Use of Therapeutic Apheresis in Clinical Practice–Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue.
- Surgery:38
- Surgical debridement
- Fasciotomy for limb threatening ischemia and compromised blood flow from tissue edema
- Amputations (below-the-knee-amputations the most common procedure)
- Skin grafting
Prognosis
- About 40%-50% mortality, mostly in the first hours after ICU admission.39
- Patients surviving PF may suffer from extensive skin necrosis and acral gangrene requiring limb amputations in almost one-third of PF survivors with a median number of three limbs amputated.40
Clinical practice guidelines
- Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology:
- In cases of DIC where thrombosis predominates, such as arterial or venous thromboembolism, severe purpura fulminans associated with acral ischemia or vascular skin infarction, therapeutic doses of heparin should be considered.
- Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines does not mention purpura fulminans.
- Underlying disorders of disseminated intravascular coagulation: Communication from the ISTH SSC Subcommittees on Disseminated Intravascular Coagulation and Perioperative and Critical Care Thrombosis and Hemostasis does not mention purpura fulminans.
- Differential diagnoses for sepsis-induced disseminated intravascular coagulation: communication from the SSC of the ISTH does not mention purpura fulminans.
- Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) mentions purpura fulminans only in reference section.
