Bottom line
- Mean platelet volume (MPV) is automatically generated by virtually all modern hematologic analyzers as part of the CBC.
- However, the MPV is not always provided to clinicians at point of care.
- The value of the MPV is limited by the lack of specific calibration and standardization guidelines.
- In clinical practice, the MPV may help in distinguishing whether:
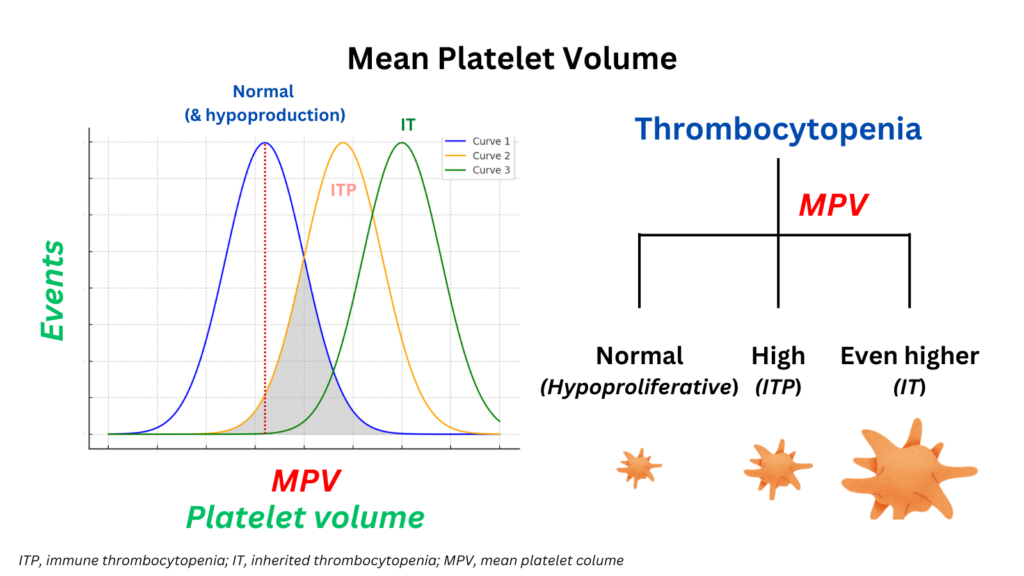
- Thrombocytopenia is due to peripheral destruction of platelets (where MPV is typically elevated because the bone marrow produces younger, larger platelets to compensate for the increased destruction of platelets in the bloodstream) or due to reduced production of platelets (where MPV is normal or low).
- Thrombocytopenia is due to ITP (elevated MVP) or inherited macrothrombocytopenias (where the MVP is even higher).
- Although the MVP may be helpful for differentiating between different causes of thrombocytopenia, it has insufficient discriminatory power to be definitive on its own.
- A number of population-level studies have reported that:
- The MPV is elevated in certain medical conditions, most notably those associated with acute thrombosis or an increased risk of thrombosis.
- An increased MPV portends a poor prognosis in certain conditions.
- However, the value of the MPV in diagnosing and prognosticating at the level individual patients with these conditions is unclear.
Introduction
- The MPV is automatically generated by virtually all modern hematologic analyzers.
- However, unlike the analogous mean corpuscular volume (MCV) of red blood cells, MPV analysis:
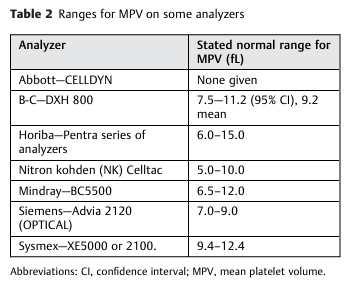
- Lacks specific calibration and standardization guidelines; as a result, normal values can vary widely between 6.0 and 13.2 fL.1
- Has a narrow dynamic range and, outside of macrothrombocytopenia, observed differences between groups are small and the affected groups typically have MPVs that are within the normal range. 2
- Is not as accurate or reproducible and has uncertain clinical significance; therefore is infrequently used for diagnostic or prognostic purposes.3
- Presently, the MVP is most frequently used for differentiating inherited macrothrombocytopenias (particularly those with giant platelets) from immune thrombocytopenia.
- The MPV has been associated with a large number of clinical conditions, and studies have shown a correlation between MPV and disease outcome, suggesting it may have underutilized clinical value.4
Definitions
- Platelets are small anucleate discoid cells originating from bone marrow megakaryocytes:5
- Each megakaryocyte can produce up to 1000–5000 platelets and, approximately 1 x 1011 platelets per day are generated under normal conditions.
- Megakaryopoiesis is regulated by the cytokine, thrombopoietin (TPO).
- After circulating for 7–10 days, platelets undergo a process of senescence characterized by the loss of their surface receptors and subsequent removal by phagocytic cells of the reticuloendothelial system.
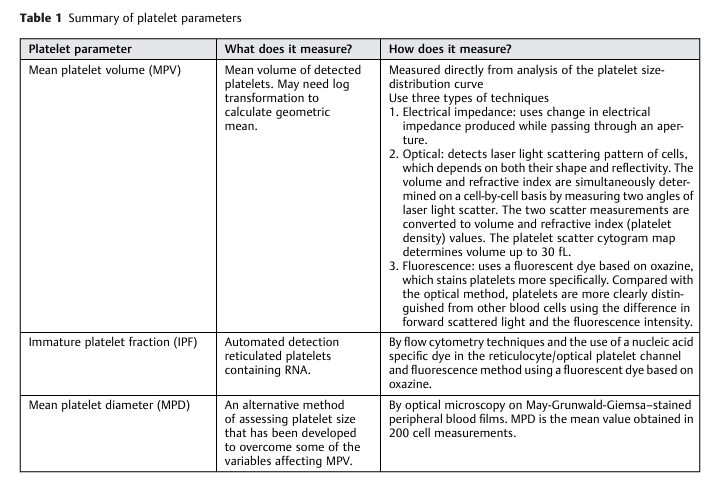
- Mean platelet volume (MPV):
- MPV is the average size of platelets, reported in femtoliters.
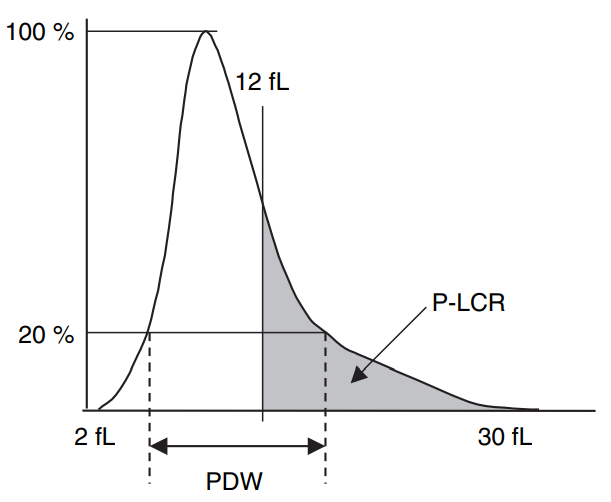
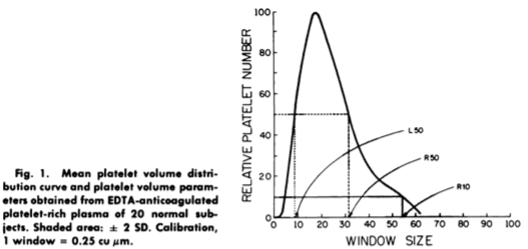
- MPV is an analyzer-calculated measure of platelet size, determined directly from analysis of the platelet size-distribution curve (see example of such a curve below). 6
- Because the MPV varies depending on the instrument used, each laboratory has a unique reference range, usually about 8 to 12 fL.7
- Platelet distribution width – refers to the volume distribution curve width at the level of 20%.8
MPV assays
- Measurements of platelet size:
- Impedance distribution (Coulter method):
- Abnormally sized platelets may be underestimated by impedance counting.
- attributed to abnormalities of platelet distribution curves9
- Optical detection systems:
- Optical light scatter, which uses laser light scatter detection at low and high angles to differentiate size (volume) and refractive index (granularity) respectively.
- Fluorescence or flow cytometry, based immunological labelling (for example using CD41 and CD61 monoclonal antibody-labeled whole blood).
- Image analysis of blood films.10
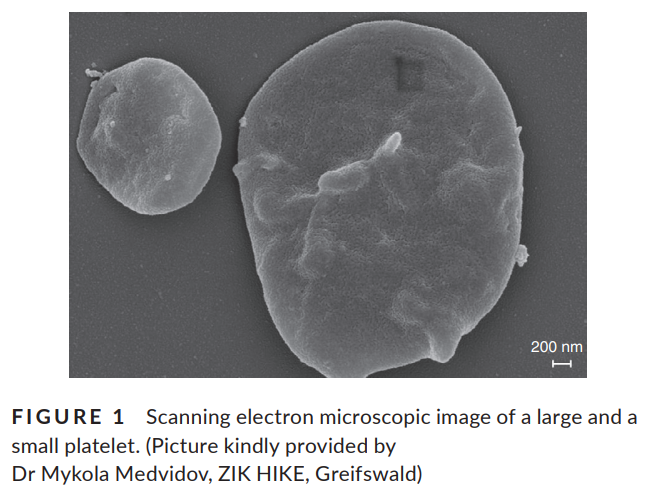
- Electron microscopy11
- Calculated from the plateletcrit and platelet count
- Impedance distribution (Coulter method):
- Advantages of the MVP:
- Noninvasive
- Simple
- Quick
- Cost-effective
- Easy-to-perform
- Reliable marker
- Disadvantages of the MPV:
- There are currently no national or international standards for measuring MPV.12
- Results vary according to technical conditions:
- Impedance vs. optical methods
- Automated counters from different manufacturers
- Moreover, the MVP is subject to numerous pre-analytical variables including:
Normal range of the MVP
- The normal range of MPV (7.7 – 10.5 fL) can vary according to:15
- Ethnicity16
- Existence of certain single nucleotide polymorphisms (SNPs)
- Platelet count:
- Inverse correlation exists between MPV and platelet count with the total platelet mass remaining fairly constant under normal conditions.17
- Normal ranges of MPV according to platelet count are not available.
- Newly formed platelets are younger and larger than normal.18
- Preanalytical variables
- The counting method used (electrical impedance vs. optical methods)
- The analyzer used
- The statistical method used
- No correlation exists with sex or age.
- The MPV remains stable in healthy individuals over the lifetime.19
- Judging whether the platelet volume of a single subject is moderately increased or reduced compared to that expected on the basis of his platelet count, age, sex, and genetic background is currently not possible.
- Within an individual, platelets are heterogeneous in size and density, which is reflected in a classical log-normal distribution of platelet size.20
Physiology and pathophysiology
- Physiological control of platelet size:21
- Under steady state platelet production thrombopoietin stimulates bone marrow megakaryocytes to generate platelets.
- Thrombopoietin is synthesized in the liver and its plasma levels are controlled by circulating platelets.
- Platelets bind thrombopoietin, which is then no longer available to stimulate thrombopoiesis.
- Removal of glycan deprived platelets via the hepatic Ashwell Morell receptor induces thrombopoietin synthesis and release in the liver.
- Megakaryocytes build pseudopodia, which form extensions that release preplatelets into the bone marrow sinusoids.
- Preplatelets convert into barbell-shaped proplatelets to form platelets.
- Platelet size is established by microtubule and actin-myosin-spectrin cortical forces which determine platelet size during the vascular formation of barbell proplatelets.
- Conversion is regulated by the diameter and thickness of the peripheral microtubule coil.
- When healthy humans receive thrombopoietin.
- Proplatelet formation is dynamic and influenced by platelet turnover, which rises upon increased platelet consumption and/or sequestration.
- A high MPV is associated with increased platelet production while a low MPV indicates decreased platelet production.22
- ITP is associated with increased platelet size:23
- Platelet life span is decreased and circulating platelets are younger.
- Platelet diameter reported to be 1.6 times higher in ITP patients.
- Lower platelet counts correlate with higher MPVs also in ITP.
- Two theories concerning correlation between MPV and thrombosis:
- MPV is the cause of vascular occlusion/thrombosis:
- When increased platelet size is an acquired characteristic, the large platelets have higher thrombotic potential (larger platelets are functionally more active):24
- Larger platelets have increased contact region on the vessel wall.
- Larger platelets express higher levels of P-selectin and glycoprotein IIb– IIIa per unit volume of platelet; larger platelets are denser, contain more secretory granules and mitochondria per unit volume of platelets, and are more reactive than their smaller counterparts.
- Studies in rabbits have shown that thromboxane B2 production/unit volume of platelet is increased in larger platelets produced after 24 hours of thrombocytopenia compared with those produced in normal steady state, concluding that platelets produced in response to thrombocytopenia not only have a larger MPV but are also more reactive.
- May explain why a high MPV has been shown to correlate with worse patient prognosis once thrombosis has occurred.
- When increased platelet size is an acquired characteristic, the large platelets have higher thrombotic potential (larger platelets are functionally more active):24
- MPV is not the cause, but instead the consequence of vascular occlusion:
- MPV is increased following platelet consumption and acceleration of platelet turnover in thrombotic conditions:25
- Activated platelets expand their ‘measured’ volume by extending pseudopodia and younger (i.e. more active) platelets have a greater MPV.
- Moreover, platelet consumption during thrombus formation accelerates platelet turnover, and this increases blood concentration of thrombopoietin, which stimulates platelet production and raises the percentage of young platelets. Young platelets have enlarged size and, therefore, thrombus formation eventually results in increased MPV.
- This may explain large platelet size of patients with arterial thrombosis: the high MPV is not the cause, but instead the consequence of vascular occlusion.
- MPV is increased following platelet consumption and acceleration of platelet turnover in thrombotic conditions:25
- MPV is the cause of vascular occlusion/thrombosis:
- The association between MPV and other acquired conditions, including hypertension, diabetes, chronic obstructive pulmonary disease, obstructive sleep apnea syndrome, obesity, and cancer may relate to the predisposition of these conditions to thrombosis; even in the absence of evident thrombotic events, may be due to abnormalities of arterial vessels and/or subclinical activation of coagulation, thus causing platelet activation-consumption and resulting in larger platelets.26
Role of MVP in diagnosis
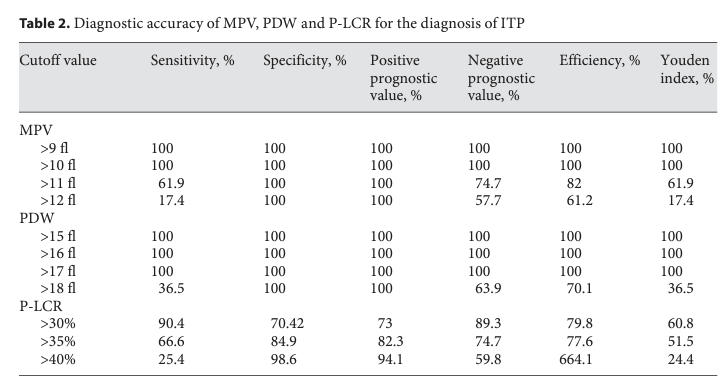
- MVP for differentiating between different causes of thrombocytopenia:
- In clinical practice the MVP is used only for the differential diagnosis of thrombocytopenic disorders.27
- Several diagnostic methods have been proposed to discriminate whether a low platelet count in a patient is caused by decreased production (e.g., inherited thrombocytopenia) or increased destruction (especially ITP):
- Bone marrow examination – gold standard
- Peripheral smear:28
- Microscopic examination of peripheral blood smear for the evaluation of platelet size.
- Recognizes platelets regardless of their size and thus makes possible accurate measurement of their diameters.
- However, this method also has drawbacks in that it is time consuming and requires experienced operators.
- Platelet-associated immunoglobulin G (PAIgG) autoantibodies:29
- Lack of sensitivity
- Not a specific test for ITP
- MPV
- ITP is associated with increased platelet size
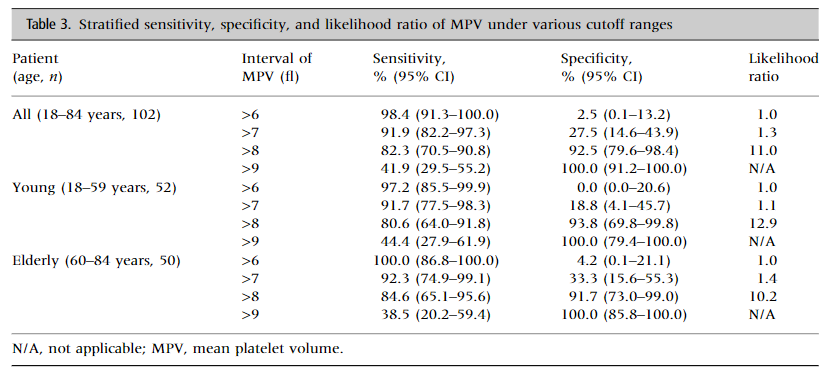
1. Use of MPV in discriminating between acquired decreased production and ITP:
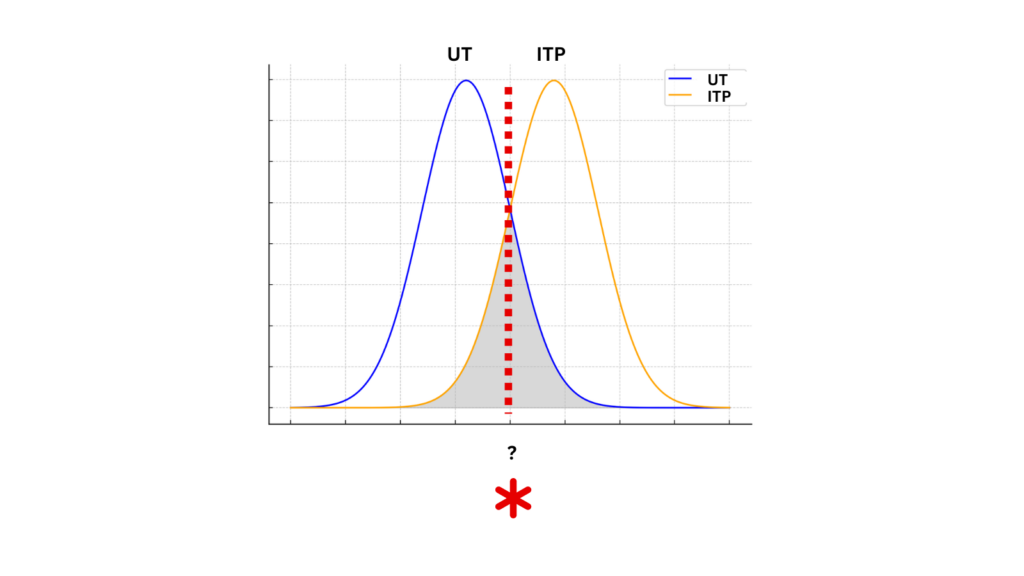
- The diagnostic accuracy of mean platelet volume in differentiating immune thrombocytopenic purpura from hypo-productive thrombocytopenia: A systematic review and meta-analysis:
- Relevant published studies that were published up to April 10, 2023, in peer-reviewed journals were searched on electronic different databases.
- A total of 14 articles were included.
- “The AUC of the SROC curve was 0.87 and the standard error was 0.0386, suggesting that it was possible to differentiate ITP from hypo-productive thrombocytopenia using MPV with excellent diagnostic performance”
- The comparison of MPV between groups revealed that the pooled mean value of MPV increased significantly in ITP patients compared to patients with hypo-productive thrombocytopenia (WMD = 2.03; 95% CI, 1.38–2.69).
- The pooled sensitivity and specificity of MPV in differentiating ITP from hypo-productive thrombocytopenia were 76.0% (95% CI: 71.0%, 80.0%) and 79.0% (95% CI: 75.0%, 83.0%), respectively.
- The summary positive likelihood ratio (PLR) and negative likelihood ratio (NLR) using the random effects model were 3.89 (95% CI: 2.49, 6.10) and 0.29 (95% CI: 0.18, 0.46), respectively.
- Conclusion: “MPV can be used to discriminate ITP from hypo-productive thrombocytopenia. It can possess large advantages as it is noninvasive, simple, quick, inexpensive, easy to perform, reliable, and routinely generated by automated cell counters.”
| PMID | Patients | Findings |
|---|---|---|
| 9883801 | 156 children; 28 had a diagnosis of ITP and 128 had a low platelet count due to decreased production (patients receiving chemotherapy or having aplastic anemia or acute leukemia) | MPV 10.02± 0.58 in ITP group, 8.05±0.96 in hypoproduction group |
| 15725092 | 40 patients with hypoproductive thrombocytopenia (aplastic anaemia; AA) and 39 patients with ITP | MPV 12.2±0.2 in ITP group, 10.2±0.2 in hypoproduction group |
| 18511864 | 63 patients with ITP, 71 with hypoproductive thrombocytopenia due to myelosuppression secondary to chemotherapy for hematological malignancies | MPV 11.38± 0.57 in ITP group, 7.17±0.54 in hypoproduction group |
| 23251270 | 124 patients with ITP, 268 with bone marrow failure | MPV 10.3±1.8 in ITP group, 9.0±1.8 in hypoproduction group |
| 27375850 | 33 patients with ITP, 50 patients had hypoproductive thrombocytopenia | MPV 12.4±3.6 in ITP group, 9.7±0.9 in hypoproduction group |
| 27926581 | 118 patients with ITP, 35 MDS patients | MPV 10.10±1.76 in ITP group, 9.4±1.31 in hypoproduction group |
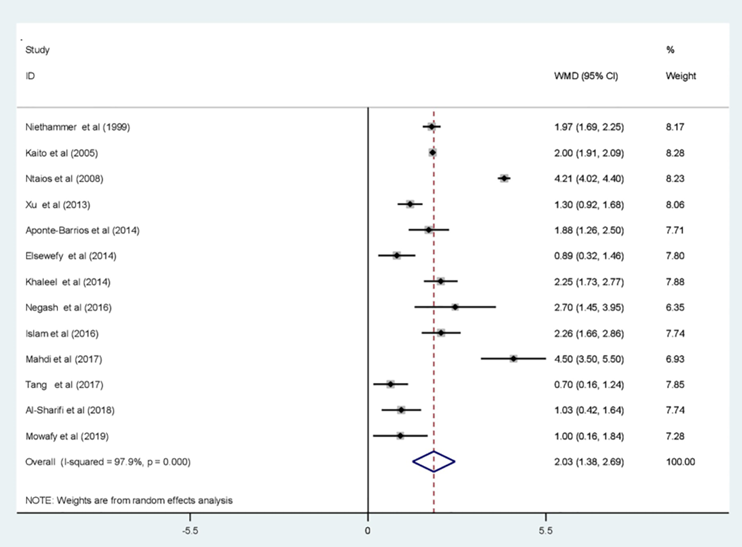
- Examples of results from individual studies:
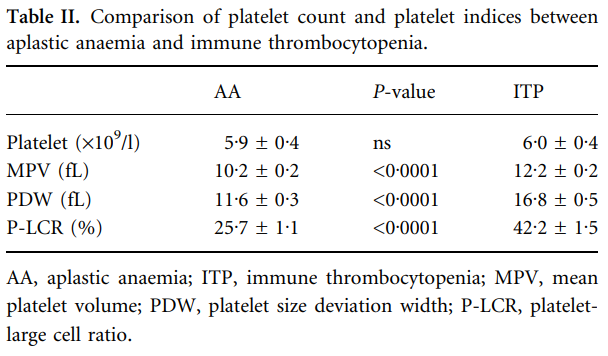
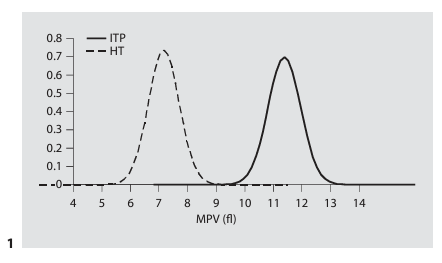
2. Use of MPV in discriminating between familial macrothrombocytopenia and ITP:
- Some studies support the use of MPV for diagnosis of ITP.
- “Differentiating ITP from inherited thrombocytopenias is not always easy as both groups have thrombocytopenia and large platelets.”30
- “The major difficulty in performing such a study was associated with the rarity of inherited thrombocytopenias and the low number of patients that are encountered in single centers. Moreover, multicenter studies have been hampered by differences in the cell counters used at different centers, making MPV values difficult to compare.31
- Examples of clinical studies:
- Noris et al, 2009
- Introduction:
- Diagnosis of ITP. relies on exclusion of alternative causes of thrombocytopenia. differential diagnosis is sometimes difficult. In particular, the differential diagnosis between ITP and inherited thrombocytopenias can challenge the clinician.
- The low platelet count of patients with constitutional forms of thrombocytopenia is recognized with increasing frequency in asymptomatic subjects during routine blood counts.
- Misdiagnosis not without consequences, as patients with inherited thrombocytopenias may undergo needless splenectomy, administration of steroids and/or intravenous immunoglobulins.
- Being misdiagnosed with ITP represents an actual risk for patients with inherited thrombocytopenias, and only the identification of simple, reliable tests for the differentiation of these conditions might reduce the number of such diagnostic errors.
- The measurement of platelet-associated immunoglobulins is considered to be a sensitive tool, but its specificity is low.
- For a long time, there has been a general consensus that the evaluation of platelet size is a useful tool for differential diagnosis, as the less rare genetic forms (i.e. MYH9-RD, and monoallelic and biallelic BSS) have very large platelets and are therefore classified as macrothrombocytopenias, whereas platelet size is only modestly increased in ITP.
- However, this concept has not been supported by experimental data, as no comparative study has been performed.
- Although guidelines may suggest that platelet size is a useful parameter for discriminating between inherited macrothrombocytopenias and ITP, no study has yet formally supported this statement.32
- Patients:
- Thirty-five patients with inherited macrothombocytopenias (15 subjects with MYH9-RD, three with biallelic BSS, and 17 with monoallelic BSS)
- 56 subjects with well-documented ITP
- 40 healthy controls
- Methods:
- MPV measured within 2 h of sampling by two different automated blood cell analyzers: the ADVIA 120 (optical scatter) and the Sysmex XE-2100 (impedance method).
- Platelet diameters were measured by optical microscopy on May–Grunwald–Giemsa-stained peripheral blood films and software-assisted image analysis.
- Results:
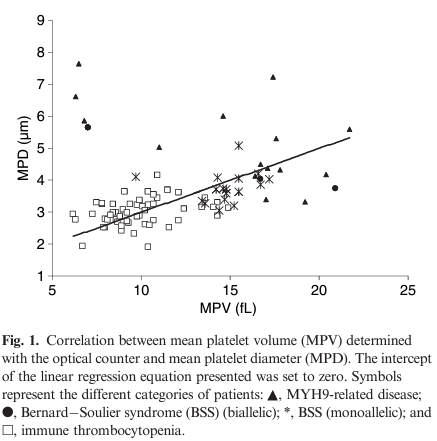
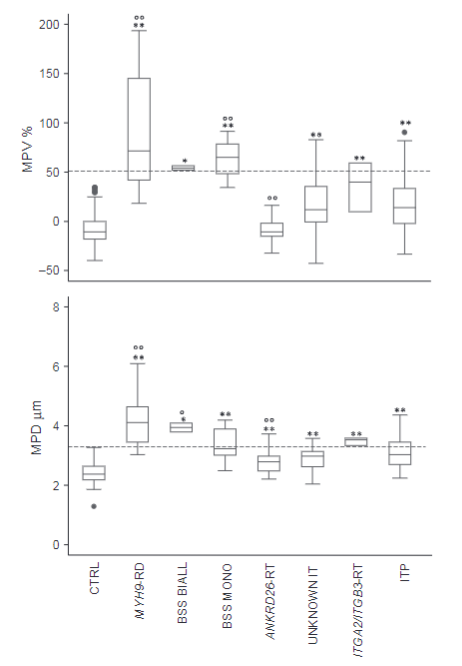
- MPVs obtained with the optical counter and MPDs measured on blood smears were much higher in MYH9-RD and both monoallelic and biallelic BSS samples than in control samples, thus confirming the definition of these disorders as macrothrombocytopenias.
- Also in ITP, MPVs and MPDs were higher than in controls, but with a large degree of overlap.
- The platelet size data placed ITP between genetic macrothrombocytopenias and healthy subjects.
- Good correlation was found between MPVs and MPDs, although a few notable exceptions were observed.33
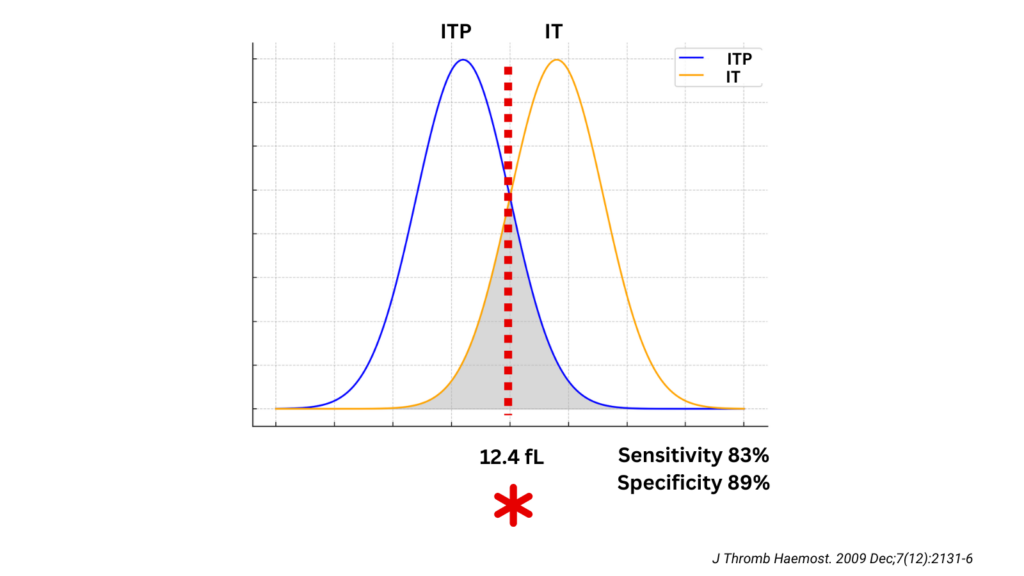
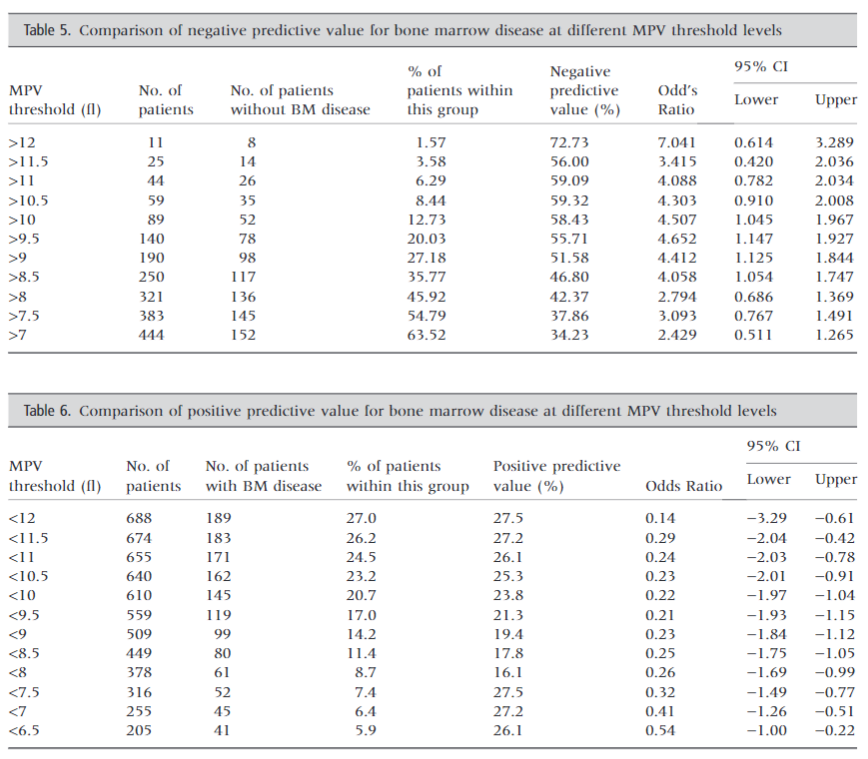
- Platelet size for differentiating inherited macrothrombocytopenias from ITP:
- With regard to MPV, the best cut-off value for distinguishing MYH9-RD/BSS from ITP was 12.4 fL (sensitivity of 0.83; specificity of 0.89),
- With regard to MPD, the best cut-off value was 3.3 um (sensitivity of 0.89; specificity of 0.88).
- The positive and negative predictive values for MPVwere0.83and0.90, whereas those for MPD were0.81 and 0.93
- Therefore, MPD was a better parameter for distinguishing between inherited thrombocytopenias and ITP.
- The best sensitivity for discriminating inherited macrothrombocytopenias from ITP was obtained by combining MPV and MPD data.
- Conclusions:
- Our study confirmed the limitation of electronic counters in recognizing very large platelets, as both impedance and optical instruments overestimated the degree of thrombocytopenia in MYH9-RD and BSS, when compared with manual counting.
- 3.3 um for MPD and 12.4 fL for MPV measured by the optical counter were identified by ROC analysis as the best cut-off values for differentiating ITP from inherited macrothrombocytopenias. Sensitivity and specificity of MPV were 83% and 89% respectively, while those of MPD were 89% and 88%.
- Discrepancies between the results from impedance and manual counting were the most relevant, and some patients with giant platelets were classified as having profound thrombocytopenia by the former method, whereas they had only a moderate decrease in platelet count by the latter.
- Limitations of cell counters in identifying large platelets resulted in underestimation of MPV in subjects with macrothrombocytopenias.
- In conclusion, we believe that combining microscope evaluation of MPDs with the value of MPVs obtained using an optical counter represents a powerful tool for distinguishing the most frequent forms of inherited macrothrombocytopenias from ITP.
- Introduction:
- Noris et al, 2009


- Examples of clinical studies (cont’d):
- Noris et al, 2013
- Introduction:
- Distinguishing immune thrombocytopenia (ITP) from inherited thrombocytopenias (ITs) is not always easy, and several patients with inherited forms have received unnecessary medical treatments or even splenectomy because they were misdiagnosed with ITP.
- Differential diagnosis may be especially difficult when a low platelet count, which can be used to decide whether thrombocytopenia is acquired or congenital, is incidentally discovered in asymptomatic subjects who have.
- Absence of affected family members by no means excludes genetic disorders because many ITs are transmitted in a recessive fashion or derive from de novo mutations.
- As many forms of IT are characterized by platelet macrocytosis, it is commonly accepted that the evaluation of platelet size is an important tool to provoke suspicion of these disorders.
- However, the accurate measurement of platelet size in IT subjects presents notable difficulties because some of the less rare forms, such as MYH9-related disease (MYH9-RD), monoallelic Bernard Soulier syndrome (BSS) and biallelic BSS, may present platelets that, due to their very large size, are not recognized by the electronic counters, which therefore underestimates not only the platelet count but also the mean platelet volume (MPV).
- Moreover, instruments operating on different principles also induce variability into the measurement of MPV in healthy subjects or in subjects with non-macrocytic thrombocytopenias, thus making difficult the direct comparison of MPV values obtained in different centers.
- A major problem for the use of platelet size in differentiating ITs from ITP was represented until recently by the lack of cut-off values for both MPV and mean platelet diameter (MPD).
- To remedy the last difficulty, a recent, monocentric study measured platelet size in patients with inherited macrothrombocytopenias and ITP by both cell counters and optical microscopy on blood films. It found that both techniques were effective in distinguishing the two conditions, in that a MPV higher than 12.4 fl measured with an optical cell counter (ADVIA 120) and a MPD larger than 3.3 um had sensitivity and specificity higher than 80%.
- To validate these cut-off values, we performed a multicenter study in a different series of patients.
- Patients:
- 113 consecutive patients with ITs
- 130 patients with ITP:
- 32 subjects had MYH9-RD
- 2 had biallelic BSS
- 12 had monoalleic BSS
- 37 had a form of autosomal dominant thrombocytopenia deriving from ANKRD26 mutation
- 3 had an autosomal dominant thrombocytopenia due to an integrin beta3 mutation
- 27 were unclassified
- Results:
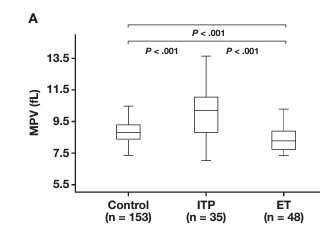
- Compared with controls, median MVP higher in:
- ITs:
- MYH9-RD
- monoallelic and biallelic BSS
- ITGA2B/ITGB3-R
- Unclassified
- ITP
- ITs:
- Compared with ITP, median MPV higher in:34
- MYH9-RD
- monoallelic BSS
- Despite these statistically significant differences, the values obtained in all ITs largely overlapped those observed in ITP.
- The already discussed cut-off values of MPV and MPD for distinguishing between inherited macrothrombocytopenias and ITP were identified by the study of subjects with MYH9-RD and monoallelic or biallelic BSS, in addition to ITP subjects:
- Limit for MPV of more than 51% with respect to controls had 91.5% specificity (vs. 89% in previous single institution study) (95% CI 84.8–95.8) and 65.9% (95% CI 50.1–79.5) sensitivity (vs. 83% in previous single institution study) in distinguishing the above IT disorders from ITP.
- Positive predictive value was 74.4% (95% CI 57.9–87)
- Negative predictive value was 87.7% (95% CI 80.5–93)
- Compared with controls, median MVP higher in:
- Conclusions:
- Platelet size is enlarged in many forms of IT and diagnostic guidelines suggest that this parameter is an important element for distinguishing these conditions from ITP. However, this statement is mainly based on expert opinion, because only one experimental study has addressed this matter in detail to date.
- The results we obtained confirm and extend many of the findings of previous investigation, but also challenge some previous conclusions.
- Confirmed the Inadequacy of the hematological analyzers (namely impedentiometric) that rely on impedance in measuring platelet parameters of subjects with enlarged platelets.
- “The limitations of some instruments used in our study explain why, in the whole patient population, the sensitivity of the cut-off value for MPV of more than 51% with respect to controls was much lower than that obtained in the previous investigation that used an ADVIA 120 analyzer.
- Our study showed that platelet size evaluation is a useful tool for distinguishing inherited macrothrombocytopenias from ITP and that the better differentiation is obtained by the use of the MPV values given by appropriate cell counters.
- From a practical point of view, we suggest that microscope evaluation of blood films and measurement of MPV by cell counters should be used in combination.
- Blood film examination enables the easy identification of very large platelets, which suggest a diagnosis of inherited macrothrombocytopenias.
- If very large platelets are not identified, a MPV increased by more than 51% with respect to controls represents an important element that supports the suspicion of a hereditary macrothrombocytopenia.
- Introduction:
- Noris et al, 2013

- Clinical practice guidelines:
- International consensus report on the investigation and management of primary immune thrombocytopenia
- In childhood ITP, mean platelet volume may be used to differentiate ITP from inherited thrombocytopenia (evidence level IIb); increased mean platelet volume can be suspected on smear if there are many large platelets
- American Society of Hematology 2019 guidelines for immune thrombocytopenia – MVP not mentioned
- Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH – separates platelet size based on analysis of peripheral smear, no mention of MPV.
- Clinical and laboratory diagnosis of heritable platelet disorders in adults and children: a British Society for Haematology Guideline:
- Mean platelet volume (MPV) is a readily available derived parameter which can be reported alongside platelet count, being inversely proportional in normal populations.
- Many inherited and acquired factors such as age, gender, ethnicity and lifestyle have been reported as influencing both platelet count and MPV.
- This is confounded by the lack of standardization of pre-analytical collection procedures such as resting time, temperature and anticoagulant impacting on platelet size.
- Modern full blood count analyzers also contribute to the variation with differences of up to 25% in MPV reported between manufacturers, probably due to differences in detection methods (impedance versus optical) and their different responses to macrothrombocytes and red cell fragments.
- Laboratories should undertake local validation of instrument-specific MPV ranges being wary of cross platform comparisons.
- As abnormal platelet size is often a distinguishing feature of heritable platelet disorders (HPD), MPV has become an important diagnostic parameter.
- Fluorescent labelling involving staining of immature red cell and platelet RNA allows further differentiation when used alongside scattered light.
- The immature platelet fraction or reticulated platelet count has proven to be a useful marker of thrombopoietic activity. It can differentiate between increased and decreased platelet production and may be significantly raised in macrothrombocytopenias, sometimes disproportionately compared to granule content.
- Pitfalls remain in all the counting techniques in patients with abnormal platelet size, shape or content. Blood film microscopy should be performed to confirm platelet numbers and identify morphological abnormalities.
- Recommendations:
- Optical or fluorescence platelet counts should be run on abnormal plots or samples with unexplained impedance-derived low platelet counts (1B).
- Blood film morphology should be examined in new cases of unexplained thrombocytopenia to document morphological abnormalities of platelets and other blood cells (1B).
- MPV should be reported routinely alongside the platelet count (1B)
- Recommendations:
- International consensus report on the investigation and management of primary immune thrombocytopenia
3. An elevated MVP has been reported in many other diseases/conditions beyond ITP and IT (see table).

- Elevated MVP has been reported to be associated with a variety of thrombotic and non-thrombotic diseases/conditions.
| Disease | PMID |
|---|---|
| Acute myocardial infarction | 27686008 |
| Stroke | 27686008 |
| Mental disorders | 37644555 |
| Heart failure | 37157842 |
| Periodontal disease | 38627719 |
| Polycystic ovary syndrome (PCOS) | 34986678 |
| Smoking | 19691485 |
| Obesity | 19691485, 30731389 |
| Hypertension | 19691485 |
| Hyperlipidemia | 19691485 |
| Oral contraceptives | 28609798 |
| Menstruation | 28609798 |
| Cancer | 27162007, 30731389 |
| Fungal sepsis | 22731700 |
| Preeclampsia | 36103491 |
| Subacute thyroiditis | 28879723 |
| Autoimmune thyroid disease | 36895561 |
| Neonatal sepsis | 32769935 |
| Laparoscopic sleeve gastrectomy | 29785471 |
| Chronic exposure to high-altitude hypoxia | 35196458 |
| Psoriasis | 27686008 |
| Varicocele | 27686008 |
| Juvenile idiopathic arthritis | 27686008 |
| Primary biliary cirrhosis | 27686008 |
| Benign prostatic hyperplasia | 27686008 |

Role of MVP in prognosis
- Elevated MPV associated with poor outcome:35
- In subjects with arterial thrombotic events
- There is no evidence that a high MPV in healthy subjects is associated with an increased thrombotic risk, while it is well documented that MPV is high in subjects with arterial thrombosis and a variety of other illnesses predisposing or not predisposing to thrombosis (see table).
| Disease/condition | PMID |
|---|---|
| Cardiovascular disease | 30950847 |
| Erectile dysfunction | 35091698 |
| Acute ischemic stroke | 35636058 |
| Heart failure | 37157842 |
| Psoriasis | 35133104 |
| Ovarian clear cell carcinoma | 37804356 |
| Aneurysmal Subarachnoid Hemorrhage | 33271484 |
| Gastric cancer | 36086726 |
| Diffuse large B-cell lymphoma (DLBCL) | 28736928 |
| Severe sepsis | 27504801 |
| Cardiac myxoma | 24246061 |
- A low MPV is associated with a poor prognosis among patients with alcohol use disorder. PMID: 37277289
