Preamble
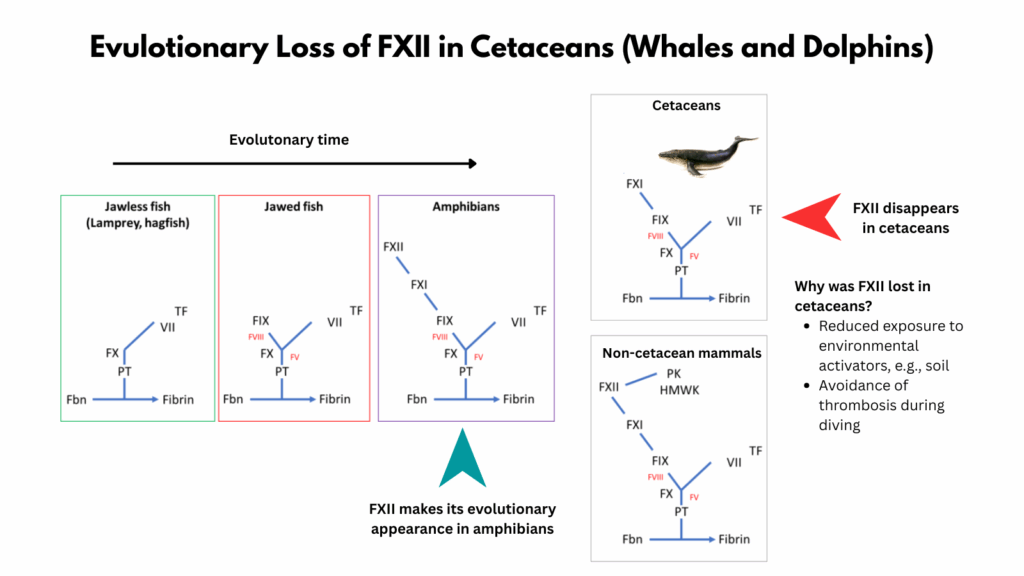
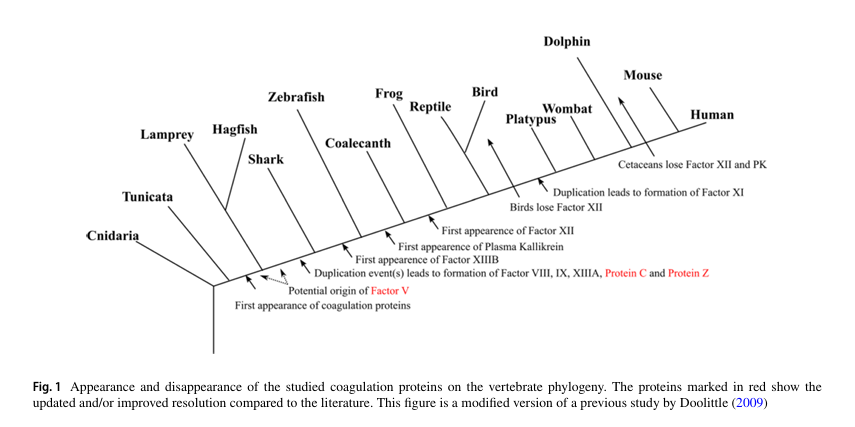
- Coagulation factor XII (FXII) first appeared in vertebrate evolution with the emergence of amphibians.
- It is absent in fish (including both lamprey and puffer fish), but is present in frogs, indicating its evolutionary origin coincides with the divergence of amphibians from earlier lineages.
- Factor XII has been independently lost in several lineages, for example, in all cetaceans (whales, dolphins, porpoises), as well as many birds and reptiles.
- The ancestors of modern cetaceans (whales, dolphins, and porpoises) transitioned from a terrestrial to a fully aquatic lifestyle during the Eocene about 50 million years ago.1
- This process constitutes one of the most marked macroevolutionary transitions in mammalian history and was accompanied by profound anatomical, physiological, and behavioral transformations that allowed cetaceans to adapt and thrive in the novel habitat.2
More about absence of FXII in cetaceans
Among marine mammals, cetaceans (which include whales, dolphins, and porpoises) are notable for lacking coagulation factor XII (FXII). This loss has been confirmed by direct biochemical studies in species such as the Atlantic bottlenose dolphin (Tursiops truncatus) and killer whale (Orcinus orca).3 Further genomic analysis has shown that both the F12 gene (encoding FXII) and KLKB1 gene (plasma prekallikrein) were lost in the cetacean stem lineage (via conversion to an inactive pseudogene), suggesting the deficiency is a general feature of all modern cetaceans (whales, dolphins, porpoises).4 In contrast, other marine mammals like manatees and most pinnipeds (seals, sea lions) retain factor XII, meaning the loss is not universal to all marine mammals, but specific to cetaceans.

It is well known that cetaceans (whales, dolphins and porpoises) are deficient in fXII, and yet these creatures seem perfectly well adapted in an evolutionary sense [32]. In this case, the history of events leading to the loss of activity is made apparent by the presence of a corresponding pseudogene. Ponczek et al, 2008
FXII is known to be missing in cetaceans… we confirmed that FXII is pseudogenized in cetaceans. According to previous studies, a pseudogene conversion by point mutations has led to this protein prediction. FXII is activated through inorganic molecules (e.g., soil). Cetaceans and birds have little to no contact to soil, therefore, it has been suggested that factor FXII has lost its importance and was subsequently lost. Coban et al, 2022
More on the biologic role of FXII
- In vitro:
- Factor XII (FXII) can initiate blood clotting in vitro when blood comes into contact with certain negatively charged surfaces, whether artificial or biological. This process, known as contact activation, involves a conformational change in the single-chain zymogen FXII upon surface binding, making it more susceptible to proteolytic cleavage. Once cleaved, FXII is converted into the active two-chain protease FXIIa, which then promotes coagulation by activating factor XI (FXI).5
- FXII-driven coagulation was first recognized as essential for surface-activated diagnostic blood coagulation assays (e.g., the activated partial thromboplastin time), which are commonly used as a clinical measure of global plasma coagulation.
- In vivo:6
- Despite its well-established role in initiating fibrin formation in vitro, coagulation FXII was long thought to be dispensable for hemostasis in vivo.
- This assumption was based on clinical observations that individuals with FXII deficiency maintain normal hemostasis and do not experience increased bleeding, even after injury or surgery.
- Similarly, FXII-deficient mice show no increase in spontaneous or trauma-induced bleeding, unlike deficiencies of most other coagulation factors. These findings suggest that FXII is not essential for physiological hemostasis.
- However, FXII-deficient mice are protected in models of thromboembolic disease, including ischemic stroke, deep vein thrombosis, and pulmonary embolism, supporting the conclusion that FXII is critical for thrombosis while largely dispensable for normal hemostasis.
- FXII also contributes to thrombosis in response to artificial surfaces and shear stress, such as those encountered in extracorporeal membrane oxygenation (ECMO) circuits.
- Juang et al. found that soil, rich in silicates, is a natural and potent activator of FXII and coagulation. In mice, soil reduced bleeding in wild-type but not FXII-deficient animals. Soil accelerated plasma clotting in a FXII-dependent manner in humans and mice, but not in species that naturally lack FXII (cetaceans, birds). The procoagulant activity of soil correlated strongly with its silicon content.7
- Beyond coagulation, FXII participates in inflammatory responses through activation of the kallikrein–kinin system, which leads to the production of the proinflammatory mediator bradykinin.8
Why was FXII lost?
One can frame the evolutionary puzzle of factor XII (FXII) in two complementary ways:
- Why has a procoagulant protein that appears dispensable for normal hemostasis been preserved in most mammals?
- A likely explanation for the evolutionary preservation of factor XII (FXII) in most mammals, despite its apparent dispensability for normal hemostasis, is that FXII contributes to coagulation under specific environmental conditions, particularly when wounds are contaminated with foreign materials such as soil. Recent studies have shown that soil, rich in silicates, acts as a natural and potent activator of FXII, accelerating clot formation in a FXII-dependent manner. This suggests that FXII may serve as a contingency mechanism, augmenting hemostasis in the presence of environmental activators that enter wounds in terrestrial settings.
- In this context, FXII likely offered a selective advantage to land-dwelling mammals, where injury often coincides with exposure to such materials. Beyond coagulation, FXII also plays roles in innate immunity and inflammation, including activation of the kallikrein–kinin system, which may further support its evolutionary retention. Thus, while dispensable in clean wounds, FXII may be adaptive in contaminated injuries, helping prevent infection or blood loss in natural environments.
- And conversely, why was it lost in cetaceans?
- In evolution, gene loss or inactivation can occur when a gene becomes non-essential, redundant, or potentially disadvantageous in a specific environment.
- Reduced exposure to environmental activators:
- On land, mammals are frequently wounded in settings where soil and other silicate-rich materials, natural activators of FXII, enter the wound and trigger coagulation. In contrast, aquatic environments lack these external FXII activators (cetaceans have little to no contact to soil), rendering the pathway less useful for hemostasis. Without environmental triggers, FXII provides little benefit.
- Avoidance of thrombosis during diving:
- Cetaceans experience prolonged apnea, peripheral vasoconstriction, and circulatory stasis during dives, conditions that increase the risk of thrombosis. Since FXII is not required for normal hemostasis but plays a key role in pathological thrombus formation, its absence likely reduces the risk of inappropriate clotting under diving-related stress.910
- Energetic and genomic economy:
- From an evolutionary perspective, genes that confer no clear benefit—or worse, pose a risk—tend to be lost over time. The F12 gene is either deleted or rendered nonfunctional in cetaceans, supporting the idea that natural selection favored its loss, particularly if it reduced the risk of thrombosis without compromising bleeding control.
- From an evolutionary perspective, genes that confer no clear benefit—or worse, pose a risk—tend to be lost over time. The F12 gene is either deleted or rendered nonfunctional in cetaceans, supporting the idea that natural selection favored its loss, particularly if it reduced the risk of thrombosis without compromising bleeding control.
FXII was likely lost in cetaceans because it offered no hemostatic advantage in an aquatic environment and posed a thrombotic risk during diving, making its retention evolutionarily disadvantageous.