Introduction
Iron deficiency anemia is among the most common problems encountered in clinical practice, yet the decision to use intravenous iron can raise practical and safety questions. This tutorial uses a real-world case to explore those questions—when and why to choose IV iron, how different formulations compare, what reactions to anticipate, and how to monitor and re-dose safely.
💡 Try the Quiz: Test your understanding of IV iron therapy after reading this tutorial. (You’ll find the link again at the end if you prefer to complete it afterward.)
Table of Contents
Case vignette
Learning setup: To bring these concepts to life, we begin with a real-world case. The details will raise a series of questions about when and how to use intravenous iron—questions we’ll explore and answer in the sections that follow.
The case: A 36-year-old woman, an otherwise well lawyer, presented to an outside emergency department with sialorrhea (ptyalism). Urgent endoscopy revealed an esophageal paper bezoar (toilet-paper foreign-body impaction) requiring prolonged endoscopic extraction. CBC showed Hb 7.8 g/dL and MCV 69 fL. The ED note did not address the microcytic anemia. Initially, when asked about toilet paper, the patient denied ingestion; with further questioning, she admitted to eating ~2 rolls/day for 3 months. Psychiatry was consulted, and she was briefly held involuntarily; recognizing that she was highly functional and otherwise well—aside from hair loss, brittle nails, and restless legs—she was discharged.
Six weeks later, she was referred to hematology. I saw her in clinic: microcytic anemia with ferritin 6 µg/L, consistent with iron-deficiency anemia (IDA) and pica (pagophagia/“xylophagia” specific to paper).
Guiding questions
Foundations
Definition / Principle
Intravenous (IV) iron refers to the parenteral administration of iron complexed with a stabilizing carbohydrate shell, which encloses the iron core and allows for controlled release to the reticuloendothelial system. It is used to correct iron deficiency and iron deficiency anemia (IDA) in patients who cannot tolerate or adequately absorb oral iron, or when rapid repletion is clinically indicated. Modern formulations have largely replaced the older, high–molecular-weight dextran products, offering safer, faster, and more convenient iron replacement.
“IV iron has traditionally been used for unresponsiveness to or intolerance of oral iron replacement therapy, or for patients for whom rapid iron replacement (for example, preoperative ID or symptomatic anemia) is desired. However, the paradigm of oral iron as first-line and IV iron as second-line therapy has taken a turn in past years because clinicians are recognizing the efficacy of IV iron over oral iron.” Ning and Zeller, 2019
Frequently Asked Questions
Before we dive into intravenous iron in depth, here’s a collection of short, focused questions that come up often in clinical practice. They’re placed right at the beginning for easy access, a way to get quick, evidence-based answers without having to read the full tutorial first. You can think of them as a pre-test or warm-up quiz, helping you check what you already know and what you might want to explore further as you move through the module. They’re presented in no particular order (they are intentionally mixed up) to keep you on your toes and make it more engaging.
IRON TRIVIA
Yes, but it is extraordinarily rare—and almost never with modern formulations.
True, IgE- or IgG-mediated anaphylaxis was historically associated with the old high-molecular-weight iron dextran preparations (no longer marketed in the U.S.). Those reactions were due to the dextran shell, not the iron itself, and sometimes occurred even with dextran solutions that contained no iron.
With today’s formulations—low-molecular-weight iron dextran (INFeD®), ferumoxytol (Feraheme®), ferric carboxymaltose (Injectafer®), ferric derisomaltose (Monoferric®), ferric gluconate (Ferrlecit®), and iron sucrose (Venofer®)—the risk of anaphylaxis is less than 1 in 200,000 infusions, and fatalities are essentially unheard of. Most reactions that appear “allergic” are actually complement-activation–related pseudoallergies (CARPA) rather than true IgE-mediated events.
When in doubt, treat first and sort later: if a patient develops airway compromise, hypotension, or generalized urticaria, manage as anaphylaxis with epinephrine and supportive care, then review the event afterward to determine whether it was true allergy or CARPA.
Bottom line: Modern IV iron can rarely cause true anaphylaxis, but the likelihood is exceedingly low. Most apparent “allergic” reactions are non-allergic, complement-mediated events, and IV iron remains one of the safest parenteral treatments in clinical use.
Modern IV iron formulations are extremely safe.
Serious hypersensitivity reactions are exceedingly rare—estimated at fewer than 1 per 200,000 infusions, with no fatalities reported for current low-molecular-weight or non-dextran products. The discontinuation of high-molecular-weight iron dextran (the older, high-risk formulation) has dramatically improved the safety profile of IV iron therapy.
The vast majority of reactions seen today are mild, transient events—typically brief flushing, warmth, chest or back pressure, or anxiety (so-called Fishbane reactions). These occur in roughly 1–3% of infusions, resolve within minutes when the infusion is paused, and do not represent allergy.
Modern pharmacovigilance studies and meta-analyses confirm that IV iron is among the safest parenteral therapies used in clinical practice, with a risk of serious reaction comparable to or lower than that of many routine intravenous medications.
In short: With current formulations and proper infusion technique, IV iron is both safe and well-tolerated. Most patients complete treatment uneventfully, and true anaphylaxis is vanishingly rare.
Management depends on the severity of the reaction.
Most reactions to IV iron are mild, self-limited CARPA-type (“Fishbane”) events that resolve quickly when the infusion is paused. The key principle is to stop the infusion, observe, and restart slowly once symptoms abate.
1. Mild (Fishbane-type) reaction
- Typical features: Flushing, warmth, chest or back pressure, anxiety, mild dyspnea.
- Action:
- Stop the infusion immediately.
- Reassure the patient; monitor vital signs.
- Symptoms usually resolve within 5–10 minutes.
- Restart at half the previous rate once asymptomatic; most patients tolerate resumption without recurrence.
- Do not premedicate with antihistamines or steroids—they provide no benefit and may confuse the picture.
2. Moderate or uncertain reaction
- Features: Persistent symptoms, mild hypotension, or diagnostic uncertainty (Fishbane vs anaphylaxis).
- Action:
- Stop the infusion.
- Give oxygen and IV fluids as needed.
- Observe closely.
- If symptoms progress or airway involvement appears, treat as anaphylaxis.
3. Severe reaction (true anaphylaxis)
- Features: Hypotension, wheeze, stridor, urticaria, angioedema, collapse.
- Action:
- Stop infusion immediately.
- Administer intramuscular epinephrine (0.3–0.5 mg of 1:1000 solution).
- Provide airway and hemodynamic support (oxygen, IV fluids).
- Add antihistamines and corticosteroids only after epinephrine.
- Document and refer for allergy evaluation before any future IV iron exposure.
Follow-up
- Document the event clearly (timing, symptoms, vital signs, management, outcome).
- Distinguish between Fishbane-type and true anaphylaxis to avoid unnecessary “iron allergy” labeling.
- Most patients who experience mild or moderate CARPA-type reactions can safely receive IV iron again with slower infusion rates.
In summary: Pause, observe, and restart slowly for mild reactions; treat promptly with epinephrine for true anaphylaxis. Most IV iron reactions are mild, non-allergic, and do not preclude future use.
- Iron sucrose
- Sodium ferric gluconate complex
- Low molecular weight iron dextran
- Ferumoxytol
- Ferric carboxymaltose
- Ferric derisomaltose
Adverse reactions to modern IV iron are rare and usually mild. They can be grouped into three mechanistic categories:
1. Complement activation–related pseudoallergy (CARPA)
- The vast majority of reactions belong here.
- Mechanism: innate immune activation from nanoparticle–complement interaction, releasing anaphylatoxins (C3a, C5a).
- Clinical spectrum:
- Mild (“Fishbane reaction”) — transient flushing, warmth, back or chest pressure, anxiety; resolves quickly when infusion paused.
- Severe (non-Fishbane CARPA) — rarer; may include hypotension, dyspnea, or syncope; still non-IgE-mediated.
- Management: stop infusion, wait for resolution, restart at half rate; do not treat routinely with antihistamines or steroids.
2. True anaphylaxis (IgE- or IgG-mediated)
- Exceptionally uncommon (< 1 per 200,000 infusions).
- Historically associated with high-molecular-weight iron dextran (no longer marketed).
- Requires epinephrine; future exposure to same product contraindicated.
3. Other rare or delayed events
- Local infusion-site reactions (extravasation, discomfort).
- Delayed arthralgia/myalgia hours later (non-allergic, self-limited).
- Hypophosphatemia with certain formulations (esp. ferric carboxymaltose).
Summary:
Most infusion reactions to IV iron are CARPA-type events, with Fishbane reactions representing their mild, transient expression and non-Fishbane reactions their more severe form. True IgE-mediated anaphylaxis is vanishingly rare with today’s non-dextran formulations.
A Fishbane reaction is a mild, transient infusion reaction that occurs in roughly 1–3% of IV iron infusions, typically within the first few minutes of administration. It is not an allergic reaction but rather a benign form of complement activation–related pseudoallergy (CARPA) — an innate immune phenomenon triggered by nanoparticles of IV iron.
Mechanism
The reaction is thought to result from transient activation of the complement system when iron–carbohydrate complexes first enter the bloodstream. This leads to the release of anaphylatoxins (C3a, C5a), which briefly cause pulmonary vasoconstriction and stimulation of afferent autonomic pathways. The result is a sudden but short-lived sensation of pressure or tightness in the chest, back, or flanks, sometimes accompanied by flushing, warmth, anxiety, or tachycardia.
Unlike IgE-mediated allergy, no antibodies, mast cell priming, or sensitization are involved — and patients are not “allergic” to IV iron.
Clinical features
- Timing: Within 1–5 minutes of starting infusion
- Symptoms: Flushing, warmth, back or chest pressure, anxiety, sometimes mild dyspnea or tachycardia
- Duration: Usually resolves within 5–10 minutes after pausing infusion
- Severity: Mild, self-limited; does not progress to anaphylaxis
- Vital signs: May show transient tachycardia or mild hypotension, but no vascular collapse, urticaria, or bronchospasm
Management
- Stop the infusion immediately.
- Reassure the patient and observe until symptoms resolve (usually within minutes).
- Restart at half the rate once asymptomatic; most patients tolerate resumption without recurrence.
- No premedication with steroids or antihistamines is necessary and may obscure the diagnosis.
- Future infusions can proceed normally, though many clinicians begin the next infusion slowly out of caution.
Clinical significance
Fishbane reactions are not a contraindication to future IV iron use. They represent the mildest expression of CARPA, distinct from both severe CARPA reactions and true anaphylaxis. Recognizing this prevents unnecessary labeling of patients as “iron allergic” and avoids withholding appropriate therapy.
IV iron is preferred when oral iron fails, isn’t tolerated, or can’t be absorbed, or when rapid repletion is clinically necessary.
Common indications include:
- Intolerance to oral iron (e.g., gastrointestinal side effects, nonadherence).
- Malabsorption (e.g., celiac disease, inflammatory bowel disease, post–bariatric surgery).
- Inflammatory states with impaired intestinal iron uptake (e.g., CKD, heart failure, chronic inflammation).
- Ongoing blood loss exceeding oral replacement capacity (e.g., heavy menstrual bleeding, GI bleeding).
- Need for rapid correction (e.g., preoperative anemia, symptomatic iron deficiency, late pregnancy).
- Functional iron deficiency in patients receiving erythropoiesis-stimulating agents (CKD, oncology).
In brief:
Oral iron is first-line for most mild, uncomplicated cases. IV iron is appropriate when oral therapy doesn’t work, isn’t feasible, or isn’t fast enough.
History of Medicine
- IM ferric oxyhydroxide:1
- First reported in 1932 in a paper by Heath et al.2
- Like today’s preparations, it contained an insoluble, colloidal complex surrounded by a carbohydrate shell, within which an Fe³⁺ core was stabilized.3
- However, the preparation released large amounts of labile free iron even at relatively low doses and was associated with numerous serious adverse events.
- The maximum tolerated was 6–32 mg.
- The intramuscular injection was painful, stained the buttock and required multiple injections.
- “Immediately following the injection and for 30 min thereafter, there was a disagreeable feeling of general warmth, palpitation, pressure in the precordium, nausea, and frequent vomiting in the patients.”
- 14 years later, it was reported that the “parenteral administration of iron is impractical, dangerous and unnecessary as a therapeutic procedure”.
- IM iron dextran:4
- 1954 paper by Baird and Padmore, reported results of the administration of intramuscular iron dextran, a complex of ferric oxyhydroxide stabilized by a dextran shell that binds iron much more tightly than earlier ferric oxyhydroxide–saccharate preparations, allowing larger doses to be administered more rapidly than ever before.
- “By the end of the 1940’s the perception of danger with intravenous iron was so widespread, it is remarkable that around that time, Baird and Padmore5 introduced a solution of HMW-ID for intramuscular injection.”5
- Painful and required frequent injections.
- IV iron dextran:6
- First reported in 1964.7
- Adverse events were uncommon but hypersensitivity reactions continued to be reported.

All IV iron complexes share the same ferric oxyhydroxide core. What evolved over time is the chemistry of the carbohydrate shell, nanoparticle control, and manufacturing precision — transforming a once-dangerous therapy into a routine, safe, and highly effective treatment.
- Then: same Fe(III)–oxyhydroxide nucleus, but crude, unstable, and immunogenic coatings.
Now: same nucleus, but highly engineered carbohydrate shells → stable, safe, single-dose formulations.
Evolution of Parenteral Iron Dextran Preparations
| Formulation | Year | Iron Core | Carbohydrate Shell | Features / Issues |
|---|---|---|---|---|
| IM ferric oxyhydroxide (“iron saccharate”) | 1932 | Fe(III) oxyhydroxide | Simple saccharate (sugar acid) | Unstable, high labile iron, painful, toxic. |
| IM iron dextran (HMW-ID) | 1954 | Fe(III) oxyhydroxide | Dextran (polysaccharide polymer) | Stable, allowed higher dosing, painful, occasional severe reactions. |
| IV iron dextran (HMW-ID → LMW-ID, e.g., Imferon®, INFeD®) | 1957–1990s | Fe(III) oxyhydroxide | Dextran (high- then low–molecular-weight polymer) | Permitted large total-dose IV infusion; high-MW form linked to frequent severe anaphylaxis; low-MW form much safer but still requires test dose. |
What Changed Over Time in IV Iron Preparations
| Feature | Early Preparations (1940s–1970s) | Modern Preparations (1990s–Present) | Why It Matters |
|---|---|---|---|
| Carbohydrate ligand chemistry | Simple or crude polysaccharides (e.g., saccharate, high–molecular-weight dextran) | Highly defined carbohydrates (sucrose, gluconate, carboxymaltose, derisomaltose, PSCME) | Determines stability, rate of iron release, immunogenicity, and risk of hypersensitivity. |
| Particle size & molecular weight | Large, heterogeneous colloids (100–500 nm) | Uniform nanoparticles (5–30 nm) with controlled distribution | Smaller, consistent particles → safer, more predictable pharmacokinetics. |
| Complex stability | Weakly bound complexes; labile iron easily released | Strongly bound, stable complexes | Reduces oxidative stress and risk of acute reactions. |
| Purity & reproducibility | Variable Fe:CHO ratio, impurities, inconsistent batches | Stringently controlled stoichiometry and manufacturing | Predictable dosing, reliable bioavailability. |
| Anaphylaxis risk | Common (esp. high-MW iron dextran) | Exceptionally rare | Modern carbohydrate ligands are non-immunogenic. |
| Dosing convenience | Small, frequent doses (100–200 mg) | Single, high-dose infusions (750–1500 mg) | Allows full repletion in one visit; improved adherence. |
| Pharmacokinetics | Rapid release → transient high transferrin saturation | Gradual macrophage processing and transferrin loading | Mimics physiologic iron handling; minimizes toxicity. |
| Regulatory and quality control | Crude colloidal suspensions | Precisely engineered nanoparticles with validated size and charge | Greater safety, uniformity, and shelf stability. |
Rationale / Advantages of IV iron Compared with Oral Iron
Oral iron remains the traditional first-line therapy for most patients with iron deficiency and iron deficiency anemia (IDA), but its effectiveness is often limited by poor absorption, gastrointestinal intolerance, and inconsistent adherence. Intravenous (IV) iron overcomes these challenges by delivering iron directly into the bloodstream, allowing rapid and predictable replenishment of total body stores and hemoglobin. It bypasses intestinal absorption barriers and avoids gastrointestinal side effects such as nausea, constipation, and abdominal discomfort.
Advantages of IV iron over oral iron include:
- Rapid and complete iron repletion: The entire iron deficit can often be corrected in one or two infusions rather than months of oral therapy.8
- Bypasses absorption barriers: Effective even in malabsorptive states such as inflammatory bowel disease, celiac disease, and after bariatric surgery, as well as in high-hepcidin conditions like inflammation or CKD.
- Improved tolerance: Avoids the gastrointestinal side effects that frequently limit adherence to oral therapy.
- Predictable dosing and adherence: Iron delivery is supervised and assured, eliminating the variability of daily pill-taking.
- Faster clinical improvement: Produces a quicker rise in hemoglobin and resolution of fatigue.
While IV iron entails higher cost, the need for infusion infrastructure, and a small but real risk of hypersensitivity reactions, serious adverse events are exceedingly uncommon with modern formulations. These tradeoffs are generally outweighed by the efficiency, tolerability, and patient satisfaction associated with IV treatment.
Increasingly, IV iron is being considered as an initial therapy, even for patients who can tolerate and absorb oral iron, when shared decision-making favors convenience, speed, or avoidance of side effects. This evolution reflects growing confidence in the safety and patient-centered value of contemporary IV iron preparations.
Although IV iron offers clear clinical advantages over oral therapy, its behavior and safety profile depend on how the iron–carbohydrate complex is processed in the body, a topic explored in the next section, Mechanism of Action.
Mechanism of Action
All modern intravenous (IV) iron products are nanoparticle colloids composed of a core of elemental iron (polynuclear Fe(III)-oxyhydroxide/oxide) surrounded by a carbohydrate shell, the nature of which varies between different preparations.9 The shell stabilizes the iron, prevents immediate release of free iron into plasma, and controls the rate at which bioavailable iron is delivered to the body. IV irons behave as prodrugs, retaining ionic iron until the iron–carbohydrate complex is metabolized.
The physicochemical differences between the IV irons include mineral composition, crystalline structure, conformation, size and molecular weight, but the key point of difference between IV iron products is the carbohydrate ligand (e.g., (e.g., sucrose, gluconate, dextran, carboxymaltose, derisomaltose, or polyglucose sorbitol carboxymethyl ether), which influences complex stability, iron release and immunogenicity, and is a unique feature of each drug.10
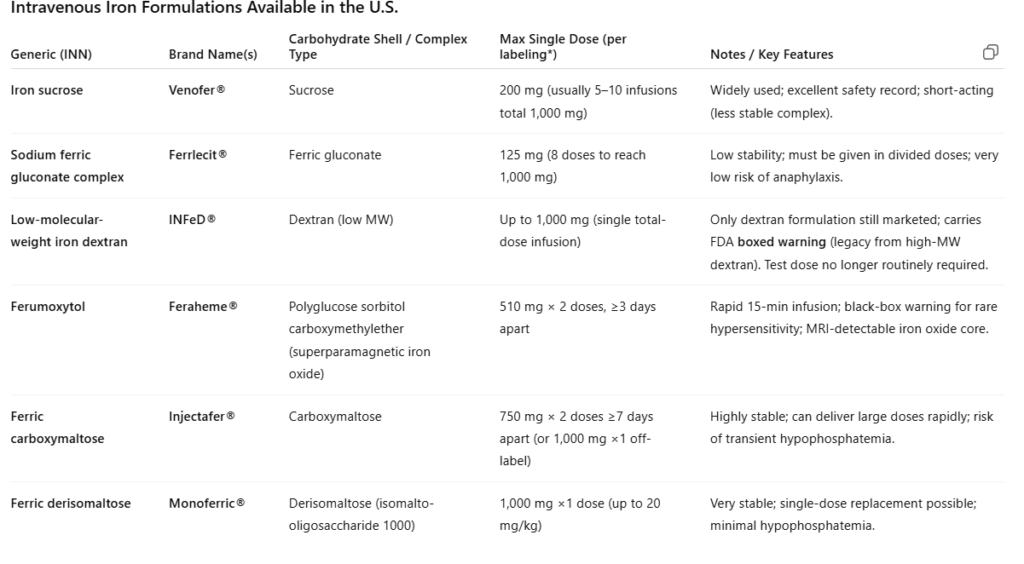
| Generic Name | Brand Name(s) | Core composition | Carbohydrate Ligand / Shell |
|---|---|---|---|
| Iron dextran (low–molecular weight) | INFeD | Fe(III) oxyhydroxide | Dextran |
| Ferric gluconate | Ferrlecit | Fe(III) oxyhydroxide | Gluconate |
| Iron sucrose | Venofer | Fe(III) oxyhydroxide | Sucrose |
| Ferric carboxymaltose | Injectafer | Fe(III) oxyhydroxide | Carboxymaltose |
| Ferric derisomaltose | Monoferric / Monofer | Fe(III) oxyhydroxide | Derisomaltose (isomalto-oligosaccharide) |
| Ferumoxytol | Feraheme | Fe(III) oxyhydroxide | Polyglucose sorbitol carboxymethyl ether (PSCME) |
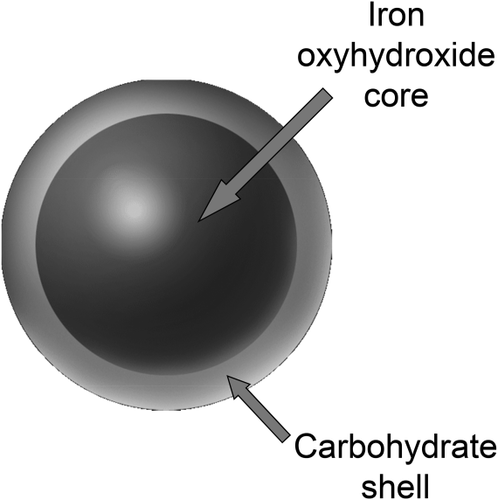
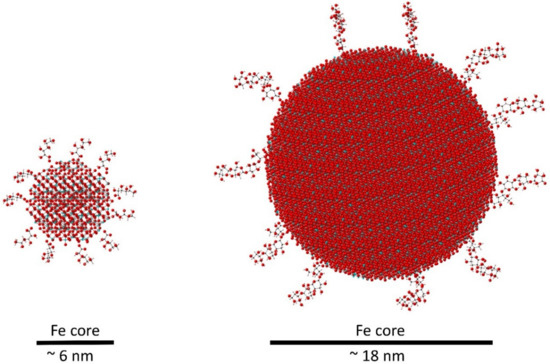
After infusion, the compound undergoes a coordinated sequence of uptake, storage, and transfer within the reticuloendothelial system (RES):
- Uptake:
- IV iron complexes are taken up primarily by macrophages of the reticuloendothelial system (liver, spleen, bone marrow).
- Uptake occurs via phagocytosis of the intact iron–carbohydrate nanoparticle.
- The iron–carbohydrate complexes bind iron tightly and do not release large amounts of labile, non-transferrin-bound iron (NTBI) into the blood before macrophage uptake.11
- Intracellular handling:
- Within macrophages, elemental iron is gradually liberated from the carbohydrate shell, which is degraded by lysosomes. The vesicles fuse with lysosomes, where the acidic, enzyme-rich environment breaks down the carbohydrate shell.12
- Iron is subsequently transported into the labile iron pool in the macrophage cytoplasm. The released ferric iron (Fe³⁺) is reduced to ferrous iron (Fe²⁺) and is then stored n ferritin or exported into the plasma by ferroportin, where it binds transferrin.
- The rate of this process is preparation-dependent—more stable complexes are degraded more slowly, resulting in more gradual iron release to ferritin and plasma, whereas less stable complexes dissociate more readily, leading to faster iron appearance in circulation.
- Importantly, only the iron (not the iron–carbohydrate complex) leaves the macrophage to enter the circulation.
- Transport:
- Once exported, iron binds to transferrin, which delivers it to the bone marrow for incorporation into hemoglobin.
- Preparation-dependent kinetics:
- The rate of iron release from macrophages depends on the stability of the iron–carbohydrate complex, which varies by formulation:
- Most stable: low-molecular-weight iron dextran (slowest release).
- Intermediate stability: iron sucrose, ferric carboxymaltose.
- Least stable: ferric gluconate (fastest release).
- The rate of iron release from macrophages depends on the stability of the iron–carbohydrate complex, which varies by formulation:
- Clinical implication:
- The more stable the complex, the slower the release and the higher the maximum single dose that can be administered safely.
Limitations / Trade-offs of IV iron Compared with Oral Iron
While IV iron has clear advantages, it also introduces distinct costs and procedural burdens that must be weighed against oral therapy. These include expense, infrastructure requirements, rare but real infusion reactions, and certain laboratory or dosing considerations. Oral iron, though less potent and less predictable, remains a simpler and safer first-line option for many patients with mild deficiency.
Limitations and trade-offs include:
- Higher cost and the need for infusion infrastructure (staff, monitoring, infusion chairs).
- Small risk of infusion or hypersensitivity reactions, though serious events are rare with modern formulations.
- Product-specific limits on total dose or infusion rate, sometimes requiring multiple sessions.
- Transient interference with iron studies (e.g., serum iron, ferritin) after infusion, which can complicate follow-up testing.
- Potential infection concerns in the setting of active systemic illness (discussed in detail below).
Compared with IV formulations, oral iron remains less expensive and easier to administer, but is often limited by gastrointestinal intolerance and poor adherence.13
| Feature | IV Iron | Oral Iron |
|---|---|---|
| Speed of repletion | Rapid, predictable rise in iron stores and hemoglobin; full correction achievable in 1–2 infusions | Slow; requires weeks to months of daily dosing |
| Absorption | Bypasses intestinal regulation and hepcidin blockade | Absorption limited by inflammation, food, and gastric acidity |
| Tolerability | No gastrointestinal side effects; rare infusion reactions | Frequent GI intolerance (nausea, constipation, abdominal pain) limits adherence |
| Adherence / Reliability | Supervised dosing ensures complete treatment | Dependent on patient compliance; often poor |
| Logistics / Infrastructure | Requires infusion facilities, monitoring staff, and scheduling | Self-administered at home; convenient |
| Cost | Higher direct and indirect cost | Inexpensive; widely available over the counter |
| Safety | Very low risk of hypersensitivity; transient lab interference post-infusion | Minimal systemic risk; possible mucosal injury or microbiome alteration |
| Use when… | Rapid correction needed, oral intolerance, malabsorption, inflammation, CKD, pregnancy, perioperative anemia | Mild iron deficiency, adequate absorption, time allows gradual correction |
- Interpretive Summary:
- IV iron offers faster, more reliable correction of iron deficiency and hemoglobin at the expense of greater cost and procedural oversight, whereas oral iron remains simpler, cheaper, but often limited by tolerance and absorption.
Patient Information: Choosing Between Oral and IV Iron
Iron helps your body make healthy red blood cells. If your iron level is low, your doctor may suggest iron pills (oral iron) or iron through a vein (IV iron). Both work to raise iron, but they are used in different situations.
Iron Pills (Oral Iron)
- Taken by mouth once or twice a day.
- Cost less and are easy to get at a pharmacy.
- Can cause stomach upset, nausea, or constipation in some people.
- Work slowly — it may take weeks or months to feel better.
- May not work well if your body has trouble absorbing iron.
Iron Through a Vein (IV Iron)
- Given in a clinic through a small IV line in your arm.
- Restores iron more quickly — often in one or two visits.
- Useful if you can’t take pills or your stomach can’t absorb iron.
- Rarely, people have mild reactions (such as flushing or a metallic taste).
- You’ll be watched closely by a nurse during and after the infusion.
How to Decide
Your doctor will help choose the option that’s safest and most effective for you. They’ll consider how low your iron is, how soon you need it corrected, and how well you tolerate pills. If you have questions, ask your care team which form fits your needs best.
💡 Bottom line: Pills are simpler and cheaper but work slowly. IV iron works faster but needs a clinic visit.
Indications and Preparations
Indications: When to Use IV Iron
Intravenous (IV) iron is not the first-line therapy for most patients with iron deficiency. However, its use has expanded steadily with greater recognition of the limitations and side effects of oral iron, the convenience of modern parenteral formulations, and the need for rapid, reliable correction of iron deficits.14 IV iron is typically reserved for situations in which oral iron is ineffective, poorly tolerated, contraindicated, or too slow to meet clinical needs.
Common Indications include:
- Intolerance to oral iron: Significant gastrointestinal side effects—nausea, constipation, abdominal discomfort—or poor adherence to daily dosing.
- Inadequate response to oral therapy (Hb < 10 g/dL by the 4th week of oral IRT.
- IDA in the second or third trimester of pregnancy.
- Malabsorption: Conditions that impair intestinal absorption of iron, such as celiac disease, inflammatory bowel disease, autoimmune gastritis, and post–bariatric surgery states.
- Inflammation and functional iron deficiency: Situations characterized by elevated hepcidin and restricted iron mobilization from stores, leading to impaired absorption and utilization. Common in chronic kidney disease, heart failure, chronic infections, and autoimmune or inflammatory disorders.
- Ongoing blood loss exceeding oral replacement capacity: Heavy menstrual bleeding, chronic gastrointestinal bleeding, or repeated phlebotomy.
- Need for rapid correction: When timely restoration of hemoglobin is essential—preoperative optimization, postpartum anemia, symptomatic severe anemia, or anemia delaying cancer therapy or surgery.
- Concomitant use of erythropoiesis-stimulating agents (ESAs): To ensure adequate iron availability for red-cell production, particularly in CKD and oncology settings.
- IRIDA
In brief: IV iron should be considered when oral iron fails, is not tolerated, or is too slow to meet clinical needs—and whenever rapid, reliable repletion of iron stores is clinically desirable. However, as with all therapies, the decision must balance risks, cost, logistics, and individual patient factors
Guideline Recommendations:
- AGA 2024:
- Intravenous iron should be used if the patient does not tolerate oral iron, ferritin levels do not improve with a trial of oral iron, or the patient has a condition in which oral iron is not likely to be absorbed.
- Intravenous iron therapy should be used in individuals who have undergone bariatric procedures, particularly those that are likely to disrupt normal duodenal iron absorption and have iron-deficiency anemia with no identifiable source of chronic gastrointestinal blood loss.
- Intravenous iron therapy should be given in individuals with inflammatory bowel disease, iron deficiency anemia, and active inflammation with compromised absorption.
- BSG 2021:
- We recommend that the initial treatment of IDA should be with one tablet per day of ferrous sulphate, fumarate or gluconate. If not tolerated, a reduced dose of one tablet every other day, alternative oral preparations or parenteral iron should be considered (evidence quality—medium, consensus—92%, statement strength—strong).
- We recommend that parenteral iron should be considered when oral iron is contraindicated, ineffective or not tolerated. This consideration should be at any early stage if oral IRT is judged unlikely to be effective (see the Treatment section), and/or the correction of IDA is particularly urgent (evidence quality—high, consensus—92%, statement strength—strong).
- EHA 2024:
- IV iron preparations consist of a carbohydrate shell with an iron core. Parenteral iron is applied in cases with moderate to severe anemia or when the response to oral iron is poor. Intravenous iron application is more efficient in improving hemoglobin values, but the higher costs of intravenous iron formulations are a clear disadvantage compared to oral preparations.
IV Iron Preparations and Formulations
Not all IV irons are created equal. They differ in chemistry, dosing capacity, infusion speed, safety, and convenience. All current preparations share a common design: a polynuclear core of ferric (Fe³⁺) oxyhydroxide encased in a carbohydrate shell that stabilizes the iron and prevents the release of toxic free iron into plasma.16 But their stability, release kinetics, and side-effect profiles vary. It is the composition of the carbohydrate shell that differentiates the iron products from each other.17
Where they differ is in the composition and structure of the carbohydrate shell, which governs the stability, rate of iron release, and likelihood of side effects.18
In brief:
- More stable complexes (e.g., ferric carboxymaltose, derisomaltose, iron dextran) can deliver larger doses in a single infusion, with slower iron release and generally lower rates of infusion-related (CARPA-type) reactions.
- Less stable complexes (e.g., ferric gluconate, iron sucrose) release iron more rapidly and may generate transient labile plasma iron, occasionally provoking mild infusion reactions, though they remain safe and widely used.
Stability influences both how IV iron behaves and how well it’s tolerated: slowing intracellular iron release (pharmacokinetics) while also reducing complement activation in plasma (safety). These effects operate through distinct mechanisms but point in the same clinical direction: greater stability means safer, smoother infusions. The safety aspect (CARPA) depends on extracellular stability. The pharmacokinetic aspect (iron appearance) depends on intracellular degradation rate.
- In plasma (before uptake):
- Stability controls how likely the complex is to prematurely release labile iron.
- More stable → minimal extracellular release → lower CARPA risk.
- Less stable → more transient labile iron → slightly higher risk of complement activation and Fishbane-type reactions.
- In macrophages (after uptake):
- Stability determines how resistant the complex is to lysosomal degradation.
- More stable → slower intracellular breakdown → gradual, sustained release of usable iron.
- Less stable → faster degradation → quicker but shorter-lived iron delivery.
Thus, while all IV irons share the same therapeutic goal (efficient, parenteral correction of iron deficiency) their stability, kinetics, and convenience differ enough that product selection should be tailored to the clinical context, setting, and patient needs.
Some of the commonly used formulations include:
Black box warnings exist fatal and serious hypersensitivity / anaphylaxis reactions for:
- Iron dextran (low-molecular-weight or historically high-molecular-weight forms)
- Ferumoxytol (Feraheme®)
BLACK BOX
No. Although all intravenous iron formulations can cause infusion reactions, their safety profiles differ. Older or less stable complexes are associated with a higher risk of hypersensitivity reactions, while newer carbohydrate shells have greatly improved tolerability.
Iron Dextran (INFeD) and Ferumoxytol (Feraheme) have black box warnings, largely because of past safety signals of serious hypersensitivity and anaphylaxis. The other IV iron formulations don’t carry boxed warnings because their risk profiles (both in clinical trials and postmarketing surveillance) have been more favorable, and no strong enough signal has required that level of regulatory warning. But the difference is not absolute — it reflects regulatory caution, historical risk, and observed reaction rates more than a mechanistic “safe vs unsafe” split.
- Iron Dextran (INFeD / older dextran forms)
- Dextran-based iron formulations historically had a higher rate of true anaphylactic (IgE-mediated) reactions, especially the older high–molecular-weight dextran.
- Because dextran itself is antigenic, even dextran-only infusions (without iron) had documented anaphylaxis cases historically.
- Low–molecular-weight iron dextran (INFeD) inherited the class label, so the warning stays: “Anaphylactic-type reactions, including fatalities, have been reported following parenteral administration of iron dextran.” FDA Access Data
- The black box is thus partly legacy, partly precaution based on historical risk.
- Ferumoxytol (Feraheme)
- After its introduction, a number of serious hypersensitivity and anaphylactic events were reported in postmarketing surveillance. U.S. Food and Drug Administration+2OptumRx Professionals+2
- The FDA responded by mandating a boxed warning indicating the risk of serious hypersensitivity / anaphylaxis and hypotension. FDA Access Data+1
- The label explicitly warns to administer slowly (≥15 minutes), monitor patients for at least 30 minutes after infusion, and have resuscitation equipment available. FDA Access Data+2FDA Access Data+2
Why other formulations don’t (yet) have black box warnings
- Their observed incidence of serious reactions has been much lower in both clinical trials and postmarketing data.
- No regulatory agency has judged that their risk justifies a boxed warning.
- Their formulations (non-dextran, more stable, smaller nanoparticle architectures) are considered inherently safer with lower likelihood of antigenic reaction or complement activation at typical infusion rates.
- For example:
- In a pharmacovigilance study, iron dextran and ferumoxytol had the highest reporting odds ratio (ROR) for hypersensitivity reactions. Iron sucrose and ferric carboxymaltose had lower RORs. PMC
- In comparative clinical use, ferumoxytol did not show a significantly higher rate of hypersensitivity/hypotension vs other compounds in some analyses, suggesting risk is low overall—but the boxed warning remains as a precaution
Key nuance & caveats
- Black box warning ≠ “unsafe” — it means extra caution is warranted, not that the drug must be avoided. Many drugs with serious risk carry boxed warnings yet are used widely under controlled conditions.
- Even in formulations without a black box, hypersensitivity or infusion reactions can still occur (though rarely).
- Regulatory labeling can lag behind mechanistic knowledge and real-world experience; a safe formulation today might acquire stronger warnings later if new signals emerge.
Summary
Iron dextran (INFeD) and ferumoxytol (Feraheme) carry boxed warnings for hypersensitivity and anaphylaxis due to historical and postmarketing safety signals. Other modern IV iron formulations lack boxed warnings because their observed rates of serious reactions have remained very low, though no IV iron is zero-risk. The absence of a black box does not imply absolute safety — rather, it reflects a balance of clinical trial data, postmarket surveillance, and regulatory judgment.
Some additional points:
- The choice between formulations often depends on how much iron needs to be given, how quickly, cost, safety, prior history of infusion reactions, and institutional or regional availability.
- Newer formulations such as ferric carboxymaltose and ferric derisomaltose have larger, more complex carbohydrate shells compared with older formulations, slowing the release of free iron and allowing for large doses of iron to be administered in a single infusion.19
- Ferric carboxymaltose and ferric derisomaltose allow large single doses, improving convenience.
- Iron sucrose and ferric gluconate are older, well-studied, and very safe but require multiple infusions.
- Ferric carboxymaltose commonly causes hypophosphatemia, whereas derisomaltose does not.
- Ferumoxytol is unique in being MRI-interfering and rapidly infused.
- In pregnancy, IV iron is generally avoided before week 13 (first trimester) because of limited safety data; later in pregnancy it may be used when needed.
- AGA 2024 Guideline: “Because there is little difference in overall efficacy of iron repletion and similar risks, formulations that can replace iron deficits with 1 to 2 infusions are preferred.”
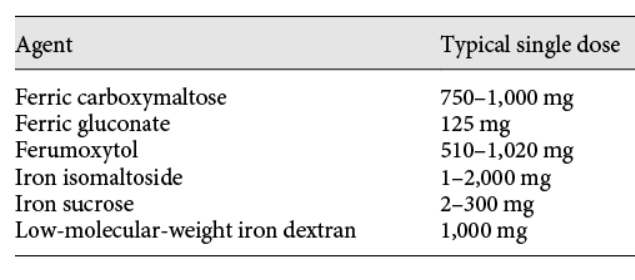
So why use IV sucrose, which requires 5 visits, over a preparation that can be given as single infusion?
- Historical and regulatory inertia:
- Iron sucrose was approved by the FDA in 2000, long before high-dose, single-visit formulations like ferric carboxymaltose (Injectafer, 2013) or ferric derisomaltose (Monoferric, 2020) were available:
- Its maximum approved dose per visit is 200 mg.
- Because most adults with iron deficiency need roughly 1 g of iron to replete stores, clinicians have to give five 200 mg doses over several weeks.
- That restriction is regulatory, not physiologic. The sucrose shell is small and less stable, so giving large single doses can release labile iron and provoke hypotension or nausea. Iron sucrose requires five visits only because of chemical and regulatory dose limits, not clinical logic.
- It’s not that we want to split the doses; we have to by label.
- Iron sucrose was approved by the FDA in 2000, long before high-dose, single-visit formulations like ferric carboxymaltose (Injectafer, 2013) or ferric derisomaltose (Monoferric, 2020) were available:
- Why some clinicians still use iron sucrose:
- Even though single-visit options exist, sucrose continues to be used because of:
- Cost and formulary: hospital purchasing contracts often favor older generics.
- Insurance coverage: some payers restrict newer formulations or require “step therapy.”
- Legacy habits: dialysis units and infusion centers are already set up for serial small-dose iron administration.
- Perceived safety: some providers (especially in nephrology) view sucrose as the “tried-and-true” option, despite higher cumulative exposure.
- Even though single-visit options exist, sucrose continues to be used because of:
- Note: giving five infusions instead of one multiplies the opportunity for a reaction fivefold. And it adds five IV starts, five chair times, five nursing resources, and so on.
- That is why modern guidelines (AGA 2024, BSG 2021, HEA 2023) explicitly state: Formulations capable of delivering total iron repletion in one or two infusions are preferred. The move toward single-visit formulations (ferric carboxymaltose, ferric derisomaltose, low-MW dextran) reflects both better chemistry and better patient experience.
Guideline Recommendation (AGA 2024):
- Intravenous iron formulations that can replace iron deficits with 1 or 2 infusions are preferred over those that require more than 2 infusions.
Dosing & Administration
The goal is to replace both circulating and storage iron. The dose can be estimated using the Ganzoni formula, but in practice, simplified tables are often used. A full replacement course usually requires 1–1.5 g of elemental iron.
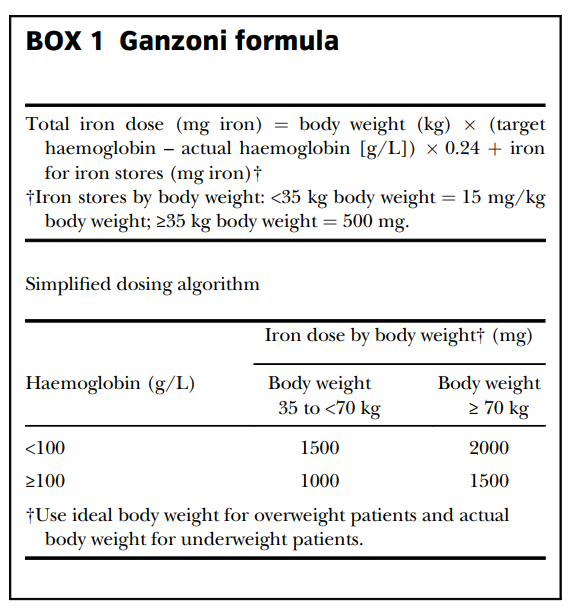
The Ganzoni equation
The Ganzoni equation (total iron deficit = body weight × (target Hb – actual Hb) × 2.4 + 500) was devised in the 1970s, when:
- Only iron dextran was available, and
- Infusions required individualized, multi-session dosing to avoid toxicity
It assumes a linear relationship between Hb rise and total iron need, which works reasonably in controlled, non-inflammatory iron deficiency but breaks down in modern practice:
- Ferritin and transferrin saturation guide dosing better than Hb.
- The equation ignores hepcidin, ongoing losses, and bioavailability differences across formulations.
- Its precision offers little advantage when iron can now be replaced safely in one or two infusions.
So while still theoretically correct, it’s clinically obsolete in most settings.
We now use “standard repletion doses”:
| Formulation | Typical dosing strategy | Rationale |
|---|---|---|
| Iron dextran (INFeD) | 1000 mg × 1 | Proven to replete total body iron in most adults without excessive labile iron exposure. |
| Ferumoxytol (Feraheme) | 510 mg × 2, 3–8 days apart | Based on PK studies showing ~1 g cumulative dose achieves comparable repletion to Ganzoni targets. |
| Ferric carboxymaltose (Injectafer) | 750 mg × 2 (1 week apart) | Same principle—empirically validated total repletion with safe kinetics. |
| Iron sucrose (Venofer) | 200 mg × 5 | Lower single-dose tolerance → divided dosing. |
Why “winging it” isn’t reckless
- When you give 1000 mg of low-molecular-weight iron dextran or 510 mg × 2 of ferumoxytol, you’re not improvising — you’re following validated, protocolized replacement dosing derived from trial data that replaced the formula:
- The variability in iron deficit between patients (often 800–1200 mg) is small compared to the safety window of modern formulations.
- The rate of iron utilization and the improvement in Hb can be monitored with follow-up labs, making precise calculation unnecessary.
- The “one-size-fits-most” approach saves time, simplifies logistics, and avoids dosing errors, without compromising outcomes.
Bottom line
Historically, total iron replacement was calculated using the Ganzoni formula, but this method has largely fallen out of use. Modern IV iron formulations have predictable pharmacokinetics and wide safety margins, allowing standardized, evidence-based dosing regimens—typically about 1 g of total iron delivered in one or two infusions. In practice, most clinicians administer fixed doses (e.g., 1000 mg of low-molecular-weight iron dextran or 510 mg × 2 of ferumoxytol) rather than calculating individualized deficits. This reflects not imprecision, but an evolution toward empiric, protocol-driven safety.
Modern formulations allow for single total-dose infusions (e.g., 1,000 mg over 15–30 minutes). Others are given in divided doses over several sessions. Patients are monitored during and after infusion for adverse reactions.
Routine premedication (e.g. antihistamines) is not recommended unless there’s a prior history of reaction. The best preventive measure is slow infusion under supervision with resuscitation supplies on hand.
- Dose calculation:
- The required total iron dose is often calculated based on body weight, current hemoglobin, and target hemoglobin (or iron deficit formula). Some protocols use simplified dosing tables.
- Some patients may require 1,000 mg or more of iron to fully replete stores; in fact, some evidence suggests 1,000 mg may still be insufficient in many patients.
- Infusion schedule / frequency:
- Depending on the preparation, iron may be given in one “total dose infusion” or in multiple smaller infusions spaced over days or weeks.
- Some formulations (e.g. ferric carboxymaltose, isomaltoside) allow for relatively large single infusions.
- The infusion rate (how fast you push the iron) is important; giving it too quickly increases risk of adverse reactions.
- After infusion, patients are usually observed for 30 to 60 minutes for signs of a reaction.
- Premedication / test dose:
- Historically, some formulations required a “test dose” (a small initial dose to assess for hypersensitivity). That is mostly relevant to older dextran formulations.
- For newer formulations, routine use of premedication (antihistamines, corticosteroids) is generally not recommended, unless there is a history of prior reaction.
- The expert consensus guidelines emphasize slow infusion and careful patient monitoring over routine premedication.
FDA Information
Infusion and Safety
What Happens During Infusion
IV iron is typically administered in an infusion clinic or outpatient center under nursing supervision. An IV catheter is inserted—usually into a forearm vein—and the iron solution is infused slowly at a controlled rate. Vital signs (blood pressure, pulse, and oxygen saturation) are monitored during the infusion and for about 30–60 minutes afterward. Most patients feel nothing unusual, though some notice a metallic taste, mild flushing, or warmth, all of which are transient. If any signs of hypersensitivity or adverse reaction arise, such as rash, shortness of breath, or hypotension, the infusion is paused or stopped immediately, and appropriate treatment (for example, epinephrine or antihistamines) is given. Serious reactions are rare, but preparedness and vigilance are key. Every infusion unit keeps emergency equipment and medications within immediate reach. Afterward, most patients can resume normal activities, though mild headache or fatigue may occur later in the day.
Step-by-Step Overview:
- Preparation: Patient screened for contraindications; IV catheter placed, often in a forearm vein.
- Infusion: Iron solution administered slowly (duration depends on product and dose).
- Monitoring: Vital signs and symptoms observed continuously and for 30–60 min post-infusion.
- If reaction signs arise: Pause/stop the infusion immediately and treat appropriately (e.g., epinephrine first for anaphylaxis; antihistamines ± steroids for urticaria/itching; oxygen/IV fluids as needed). Escalate per protocol.
- Possible sensations: Metallic taste, warmth, or flushing—brief and benign.
- Emergency readiness: Staff equipped with epinephrine and resuscitation tools for rare hypersensitivity events.
- Post-infusion: Patient discharged once stable; may resume usual activities.
What to Expect During an IV Iron Infusion
Patient Information Summary
IV iron is given in a clinic or infusion center by trained nurses.
Here’s what typically happens:
- Getting started: A small IV line is placed into a vein, usually in your arm or hand.
- During the infusion: The iron medicine drips slowly into your bloodstream while nurses check your blood pressure, pulse, and oxygen.
- How you might feel: Most people feel normal. A few notice a metallic taste, warmth, or mild flushing that fades quickly. Tell your nurse right away if you feel short of breath or unwell.
- After the infusion: You’ll be watched for 30–60 minutes, then can usually go home. Mild headache or tiredness can happen but are short-lived.
- Safety: Serious reactions are very rare. The clinic keeps emergency medicines (like epinephrine) and equipment close by—just in case.
💬 Summary:
IV iron infusions are safe and simple. Most people feel fine during treatment, and it helps restore iron and energy more quickly than pills.
Efficacy & Expected Response
Hemoglobin typically rises within 2–4 weeks of infusion, often by 1 g/dL per week if bleeding is controlled. Ferritin and transferrin saturation recover first, followed by symptomatic improvement: less fatigue, better exercise tolerance, and clearer thinking.
- TSAT:
- Spikes within hours–1 day, then settles over 3–7 days toward a new baseline (often 20–30% depending on deficit/inflammation).
- Initial response: TSAT rises rapidly and dramatically, peaking within hours to 1 day after infusion. Because TSAT reflects the fraction of transferrin bound to iron, it increases as newly infused iron transiently circulates in plasma before uptake by macrophages or tissues.
- Time course: The peak is followed by a rapid decline over 3–7 days as iron is cleared from plasma, stored as ferritin, or incorporated into red blood cells. The post-infusion baseline typically stabilizes around 20–30%, depending on the patient’s iron deficit and degree of inflammation.
- Monitoring note: TSAT is not reliable immediately after infusion due to this transient spike. Most guidelines recommend waiting at least 48 hours to 1 week—and sometimes longer—to obtain a meaningful post-treatment value.
- Ferritin:
- Rises within days, often peaks at ~1–2 weeks, then drifts down over 4–8+ weeks to a higher steady level than baseline.
- Initial response: Ferritin begins to rise within days after infusion as iron is rapidly taken up by the liver and reticuloendothelial macrophages for storage. The increase is brisk but slightly slower than the TSAT spike, reflecting redistribution rather than circulating transport.
- Time course: Serum ferritin typically peaks at about 1–2 weeks, then gradually declines over 4–8 weeks to a new, higher steady state as stored iron is mobilized for hemoglobin synthesis.
- Monitoring note: Ferritin values are artificially elevated immediately after infusion, owing to both increased iron stores and transient iron in the plasma compartment. For a reliable assessment of repletion, ferritin should be rechecked 4–8 weeks after the final infusion.
- Hemoglobin:
- Initial response: The rise in hemoglobin is delayed and gradual, reflecting the time required for erythropoiesis—the production of new red blood cells. Unlike TSAT and ferritin, which respond within days, hemoglobin increases only after iron has been incorporated into newly synthesized erythrocytes. Reticulocytosis begins in ~5 days, Hb rises by ~1 g/dL every 2–3 weeks, typically peaking at 4–8 weeks.
- Time course: A measurable increase usually begins within 1–2 weeks after infusion. A clinically significant rise of 1–2 g/dL is typically observed by 4–8 weeks, though the full therapeutic effect may take up to 2–3 months to be realized. The rate of increase depends on baseline iron deficit, ongoing blood loss, inflammation, and concurrent use of erythropoiesis-stimulating agents.21
- Significance: Hemoglobin is the ultimate clinical marker of efficacy in treating iron deficiency anemia. Symptomatic improvement—reduced fatigue, weakness, and dyspnea—often parallels the hemoglobin rise.
- Expected outcome: Once repleted, IV iron therapy typically raises hemoglobin by 20–30 g/L (2–3 g/dL) over about 8 weeks, depending on the patient’s baseline and comorbid conditions.22
The time-course response of ferritin, TSAT (Transferrin Saturation), and Hemoglobin (Hb) to intravenous (IV) iron infusions follows a predictable pattern, though the exact magnitude and duration can vary based on the iron formulation, dose, patient’s underlying condition (e.g., kidney disease, heart failure), and initial iron status.
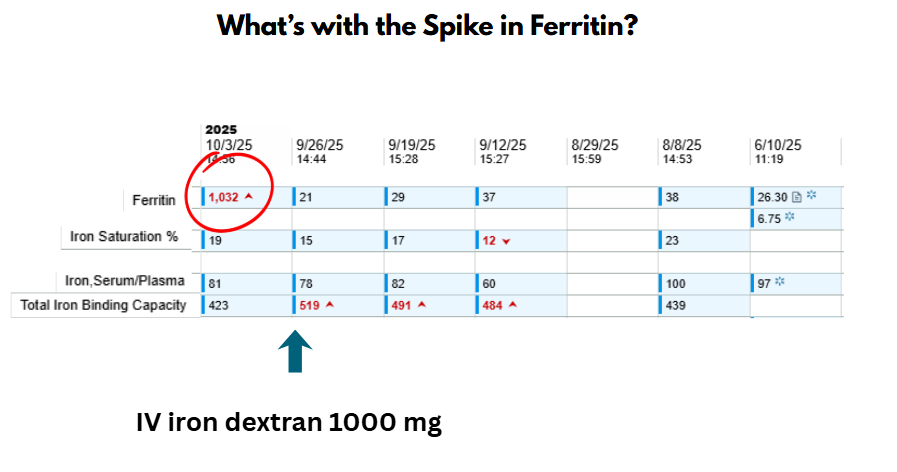
Ferritin Spike
After intravenous iron infusion, serum ferritin rises sharply within 24–48 hours, reflecting the large iron load taken up by macrophages and stored as ferritin. Transferrin saturation (TSAT) may increase modestly or remain normal, since only a fraction of infused iron is immediately released into plasma for binding to transferrin. Over subsequent days to weeks, ferritin gradually declines as stored iron is mobilized to transferrin and used for erythropoiesis.
After intravenous iron administration, serum ferritin often rises sharply for several days. This is not a reflection of iron overload but rather the expected physiologic handling of IV iron by the reticuloendothelial system:
- Iron uptake by macrophages:
IV iron complexes (e.g., iron dextran, ferric carboxymaltose) are taken up by macrophages of the liver, spleen, and bone marrow. - Intracellular ferritin synthesis:
Within macrophages, iron is stored in ferritin molecules — an immediate buffering response to the sudden influx of elemental iron. - Ferritin release into plasma:
Some ferritin is secreted into circulation, leading to a transient rise in serum ferritin levels.
Meanwhile, hepcidin increases, reducing iron export through ferroportin. As a result, transferrin saturation (TSAT) remains in the normal range despite the ferritin spike. Over the following weeks, iron is slowly released from macrophages and incorporated into erythropoiesis, and ferritin levels gradually decline toward a new steady state.

Risks, Side Effects & Safety Considerations
“While the use of parenteral iron in some providers’ minds is associated with great risks, recent studies show these are markedly overstated… Studies have shown all iron products have a good safety record, with a lower rate of reactions than rituximab or penicillin. Modern iron formulations are associated with a low risk of reactions, and they have fewer adverse effects than oral iron in several studies.” Thomas G DeLoughery, 2019
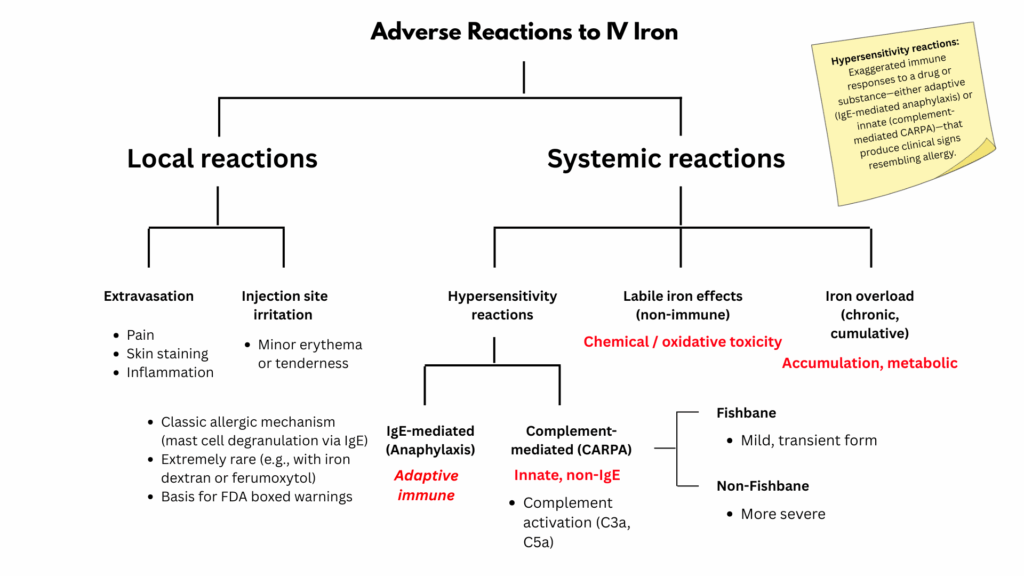
Not included in this scheme are delayed, mild hypersensitivity (or inflammatory) reactions.
1) Hypersensitivity reactions
Most infusion reactions to IV iron are not true allergies but brief, complement-activation events triggered by the nanoparticle surface of the iron–carbohydrate complex. Severe reactions are rare, and nearly all modern formulations are safe when given at recommended rates.
- True anaphylaxis:
- Most reactions are complement-activation–related pseudoallergies (CARPA)
- Incidence ≈ 1 in 200
- Brief, innate immune responses caused by carbohydrate component of the iron-carbohydrate complex (not true IgE-mediated allergy))
- Two types based on clicnial severity:
- Fishbane reaction: mild, transient flushing, warmth, or chest/back pressure; resolves quickly and is usually safe to restart at a slower rate.
- Severe (non-Fishbane) CARPA: rarer; may include hypotension, dyspnea, or more systemic symptoms. Stop the infusion, give supportive care, and do not rechallenge with the same formulation.
- Rate matters: faster infusions increase complement activation risk.
- Premedication is not routinely needed; diphenhydramine may worsen hypotension and confusion with true allergy.
- Overall, pooled data from large meta-analyses and pharmacovigilance reviews (e.g., Mayo Clin Proc 2015) show no excess in serious adverse events or infections with IV iron compared with placebo or oral therapy. Serious hypersensitivity remains exceedingly rare with modern formulations.
FISHBANE
Mechanistically, they’re on the same spectrum
- Both so-called Fishbane and non-Fishbane reactions:
- Occur within minutes of starting IV iron.
- Are non-IgE, complement-driven, innate immune events.
- Are rate-dependent and idiosyncratic.
- Share the same core mechanism: transient C3a/C5a generation leading to vasodilation, tachycardia, dyspnea, and anxiety.
- The only real difference is the magnitude of this activation and the extent of physiologic response.
So mechanistically, they are not two different entities—just different magnitudes of the same process. Same mechanism, different intensity
There’s no hard mechanistic line between a “Fishbane reaction” and a “non-Fishbane CARPA reaction.” The difference is operational, not biologic.
- Why we still label one as “Fishbane”:
- The term Fishbane reaction persists because it communicates prognosis and safety, not mechanism.
- Fishbane (mild CARPA)
- Brief, self-limited
- Stop, reassure, restart slowly
- Safe to rechallenge
- Non-Fishbane (moderate/severe CARPA)
- Hypotension, hypoxia, collapse
- Stop permanently, supportive care
- Avoid rechallenge; use alternative formulation
- At the bedside, that distinction answers the question “Can I safely restart this infusion?”
- That’s the true utility—not in mechanistic purity, but in risk stratification.
- But in clinical practice, the binary classification (mild vs severe) is more actionable than debating whether it’s “Fishbane” or “CARPA.”
- Fishbane (mild CARPA)
- The term Fishbane reaction persists because it communicates prognosis and safety, not mechanism.
- How most experts handle it in practice:
- At the bedside, most hematologists and infusion nurses now treat the terminology pragmatically:
- If symptoms are mild and resolve promptly → call it a Fishbane (mild infusion) reaction and restart slowly.
- If symptoms are more significant (hypotension, hypoxia, persistent distress) → call it hypersensitivity/infusion reaction (CARPA-like) and do not rechallenge.
- In either case, do not label the patient “allergic to IV iron” unless there’s evidence of true anaphylaxis.
- At the bedside, most hematologists and infusion nurses now treat the terminology pragmatically:
- Clinically, the distinction between Fishbane and non-Fishbane reactions is one of severity, not mechanism.
- Both are complement-mediated pseudoallergies; the “Fishbane” label simply signals the benign, self-limited end of that spectrum—important mainly to justify safe continuation of therapy.
- Fishbane and non-Fishbane CARPA reactions share a common complement-mediated mechanism. The only clinically meaningful distinction is severity — the presence of hypotension, hypoxia, or collapse transforms a benign, self-limited Fishbane episode into a more serious infusion reaction requiring discontinuation
- Clinically convenient distinction:
- “Fishbane” → safe to restart once symptoms resolve.
- “Severe CARPA” → stop permanently, switch formulation.
- That’s really the bedside decision point.
Guideline & Evidence Summary
- All intravenous iron formulations have similar risks; true anaphylaxis is very rare. The vast majority of reactions to intravenous iron are complement activation–related pseudo-allergy (infusion reactions) and should be treated as such.
- Among >10,000 infusions analyzed across 103 trials, serious hypersensitivity reactions were rare (<0.1%).
- Non-serious infusion reactions occurred in ~1–3% of infusions, consistent with complement-activation phenomena.
- No formulation was associated with a significantly higher rate of serious events.
2) Delayed post-infusion inflammatory reactions
Mild, flu-like symptoms such as headache, myalgia, arthralgia, or low-grade fever may occur 1–2 days after IV iron administration. These are self-limited inflammatory responses, not true hypersensitivity reactions. They resolve spontaneously within 24–48 hours and rarely recur with subsequent infusions.
3) Hypophosphatemia
A transient drop in serum phosphate may occur after certain IV iron formulations—most notably ferric carboxymaltose—due to elevated FGF-23–mediated phosphate wasting. While often asymptomatic, severe hypophosphatemia can develop with repeated dosing or in predisposed patients. Monitoring is recommended in patients with bone disease, malnutrition, or persistent post-infusion fatigue.
4) Infection risk
While IV iron theoretically provides a substrate for microbial growth, clinical evidence does not show a meaningful increase in infection risk with modern formulations. Caution is advised in patients with active systemic infection, but IV iron can be safely administered once infection is controlled.
Thomas G DeLoughery, 2019: “Given the role of free iron in promoting the growth of pathogenic microorganisms, there have been concerns that intravenous iron may predispose to infections. Reviews and meta-analyses have shown no increased risk of infections with intravenous iron. The recent PIVOTAL study also showed no increased risk of infection with aggressive intravenous iron supplementation in dialysis patients. In addition, compared to oral iron, intravenous iron did not lead to adverse changes in bowel microbiome in patients with inflammatory bowel disease.
5) Local / other side effects:
Most IV iron infusions are well tolerated. When side effects occur, they are usually local or transient systemic reactions—such as mild pain at the injection site, flushing, or headache—and rarely serious with modern formulations.
6) Iron toxicity
IV iron formulations are designed to deliver tightly bound, non-reactive iron, minimizing the risk of free iron–mediated oxidative injury. Transient elevations in labile plasma iron may occur briefly after infusion but are well below toxic thresholds with current preparations. Free iron toxicity is mechanistically important but clinically negligible with current IV iron preparations.
7) When premedication may be considered:
- Prior mild infusion reaction (e.g., flushing, chest pressure, “Fishbane reaction”)
- → Usually no premedication needed; slow the infusion rate, ensure close observation, and reassure patient.
- Prior moderate or unclear reaction
- → May consider:
- Methylprednisolone 40–125 mg IV (given 30 minutes before)
- H1 antihistamine (e.g., cetirizine 10 mg PO or diphenhydramine 25–50 mg IV/PO)
- ± H2 blocker (e.g., famotidine 20 mg IV/PO)
- Infuse at half rate initially, with close monitoring.
- → May consider:
- History of severe anaphylaxis to iron dextran or other formulation
- → Avoid that formulation entirely; switch to a different IV iron (cross-reactivity is low).
- → If must re-challenge, do so in a monitored setting with full resuscitation capability and premedication as above.
Monitoring & Follow-up
- Baseline evaluation:
- Before infusion, obtain iron studies (ferritin, transferrin saturation [TSAT], serum iron, total iron-binding capacity [TIBC]) and hemoglobin. A complete blood count, renal function, and phosphate are often included to assess for comorbid contributors or risks (e.g., hypophosphatemia).
- During infusion:
- Monitor vital signs and patient-reported symptoms. Watch for rash, dyspnea, chest or back tightness, and hypotension—potential signs of hypersensitivity or complement activation–related pseudoallergy (CARPA).
- Immediate post-infusion observation:
- Observe for 30–60 minutes after completion for delayed infusion reactions. Provide reassurance and education about expected transient symptoms (warmth, flushing, mild arthralgia).
- Follow-up labs & timing:
- Timing: Hemoglobin and iron studies are typically rechecked in 2–4 weeks to confirm hematologic response.
- Interpretation caveat: Serum iron and TSAT are artificially elevated immediately after infusion; most guidelines recommend waiting at least 1–2 weeks, and preferably ~4 weeks after a total-dose infusion, to obtain meaningful post-treatment values.
- Ferritin: Expect peak at ~1–2 weeks, then gradual decline over 4–8 weeks.
- Phosphate: Monitor in patients at risk for hypophosphatemia (particularly those receiving ferric carboxymaltose).
- When ferritin remains low after IV iron:
- Occasionally, ferritin remains <30 µg/L 4–6 weeks after infusion. This may indicate ongoing blood loss, incomplete repletion, or inflammatory redistribution rather than failed uptake.
- Recheck TSAT and CRP: if both are low, repeat infusion is reasonable, typically a full 1 g course if losses are ongoing, or a smaller 500 mg “top-up” for partial deficiency. Evaluate for occult bleeding or chronic inflammation before assuming treatment failure.
- Clinical monitoring:
- Assess for symptomatic improvement—fatigue, dyspnea, and exercise tolerance—and for any adverse effects or new symptoms suggestive of delayed inflammatory reactions (e.g., transient myalgia, headache, or joint pain).
- Long-term follow-up:
- Patients with ongoing blood loss, malabsorption, or chronic inflammatory disease often require periodic iron repletion. Re-treat when ferritin <30 µg/L (or <100 µg/L in inflammatory states) or TSAT <20%, even before anemia recurs. Investigate and address the underlying cause of iron deficiency (e.g., gastrointestinal bleeding, heavy menses, celiac disease) to prevent recurrence.
Maintenance and “Top-up” Dosing
After initial repletion and follow-up, attention shifts to long-term maintenance. Iron stores often decline gradually over months, particularly in patients with ongoing losses or chronic disease.
After successful repletion, ferritin may gradually decline over months as iron is utilized or lost. Re-treatment is reasonable when ferritin falls below ~30 µg/L (or <100 µg/L in inflammatory states) or TSAT <20%, even before anemia recurs. In such cases, a full 1 g replacement course is typically given, though smaller maintenance doses can be considered for ongoing low-level losses or chronic disease.
Evidence and Guidelines
Key Randomized Controlled Trials
Meta-Analyses
Clinical Practice Guidelines
Special Populations & Considerations
- Pregnancy:
- Iron deficiency affects up to 40% of pregnancies.
- Oral iron is often poorly tolerated.
- IV iron provides faster correction and improved maternal wellbeing.
- Use is generally avoided in the first trimester (before ~13 weeks) due to limited safety data.
- In the second and third trimesters, IV iron may be used when needed (e.g. severe anemia, intolerance of oral iron).
- In pregnant women, intravenous iron was more efficacious in raising blood count with a significantly reduced risk of side effects – odds ratio 0.35.29
- Heart failure:
- Iron deficiency (ferritin <100 µg/L or 100–299 µg/L with TSAT <20%) occurs in up to 50% of patients with chronic heart failure.
- Mechanisms include chronic inflammation, hepcidin upregulation, and reduced intestinal absorption.
- IV iron (ferric carboxymaltose or ferric derisomaltose) improves exercise capacity, symptoms, and quality of life.
- Several RCTs (FAIR-HF, CONFIRM-HF, IRONMAN) show reduced hospitalizations for heart failure, though mortality benefit remains uncertain.
- Oral iron is ineffective because of impaired absorption and inflammation-related iron sequestration.
- Chronic Kidney Disease (CKD) and Dialysis:
- Iron deficiency is common in CKD due to reduced absorption, inflammation-induced hepcidin elevation, and ongoing blood loss (e.g., dialysis circuits, phlebotomy).
- Oral iron is often ineffective because of poor gastrointestinal absorption and hepcidin blockade.
- Intravenous iron is the preferred route to replenish stores efficiently and support erythropoiesis-stimulating agent (ESA) therapy.
- Ferritin and TSAT targets: KDIGO suggests ferritin <100 µg/L (non-dialysis) or <200 µg/L (dialysis) and TSAT <20% as thresholds for replacement.
- Typical goal: Maintain ferritin <700 µg/L and TSAT <40%.
- IV iron improves hemoglobin response, reduces ESA dose requirements, and is safe when given judiciously, even at higher ferritin levels in functional deficiency.
- Cancer:
- Iron deficiency and anemia are frequent in malignancy due to chronic inflammation, bleeding, nutritional deficiency, and chemotherapy-induced myelosuppression.
- Functional iron deficiency (normal or high ferritin, low TSAT) is common from cytokine-driven hepcidin excess.
- IV iron, especially when combined with ESAs, enhances hemoglobin response and decreases transfusion requirements.
- Stand-alone IV iron can improve anemia even without ESA use, particularly when oral iron fails or is contraindicated.
- Evidence supports safety across cancer subtypes, though care is needed in patients with active infection.
- Surgery / Perioperative Setting:
- Preoperative iron deficiency anemia is strongly associated with increased transfusions, complications, and delayed recovery.
- Oral iron rarely normalizes hemoglobin before surgery because of limited absorption and time constraints.
- IV iron (e.g., ferric carboxymaltose, derisomaltose) provides faster correction of hemoglobin and iron stores.
- Best results occur when given ≥2–3 weeks before surgery to allow erythropoietic response.
- Some trials show improved postoperative outcomes and reduced transfusions, though results vary by population (e.g., FIT, PREVENTT).
- Pediatrics / children:
- Some IV iron formulations (especially low molecular weight dextran) have been used in children (as young as a few months, in select settings).
- But usage is more limited; pediatric dosing, safety, and protocols are more specialized.
Evidence and Safety in Special Populations
- Most of the large randomized trials evaluating modern intravenous iron formulations have been conducted in specific clinical populations—most notably in chronic kidney disease (CKD) (e.g., FIND-CKD, REVOKE), chronic heart failure (e.g., FAIR-HF, CONFIRM-HF, IRONMAN), and pregnancy. These studies consistently demonstrate that IV iron, when administered appropriately, is effective and well tolerated, with a very low incidence of serious hypersensitivity reactions. Although these populations differ from the typical patient with absolute iron deficiency anemia (such as a menstruating woman or an individual with gastrointestinal blood loss), the formulations, dosing strategies, and infusion protocols are the same. Thus, while disease-specific thresholds and treatment goals may vary, these large trials provide reassuring safety data and broad physiologic validation for IV iron use across diverse clinical contexts.
Practical & Clinical Considerations
- Cost and logistics:
- IV iron therapy is more resource-intensive (infusion facility, nursing time, monitoring) and costlier than oral iron. The benefit must justify those costs in context (e.g. when oral therapy fails or is not feasible).
- Patient selection:
- Not everyone with iron deficiency needs IV iron; many patients do well with oral therapy. Selection should consider severity of deficiency, comorbidities, GI absorption, side effects, and urgency.
- Institutional protocols / guidelines:
- Different institutions or regional guidelines may prefer certain formulations or dosing regimens. It’s important to follow evidence-based protocols and safety guidelines.
- Educating patients:
- Patients should be informed of risks, benefits, what to expect during infusion, signs to report (e.g. rash, breathing difficulty), and the need for follow-up labs.
- Addressing underlying causes:
- Treating iron deficiency is only part of the solution; it’s important to identify and manage the root cause (e.g. bleeding sources, dietary deficiencies, malabsorption).
- Documentation & safety preparedness:
- Because of the small but real risk of hypersensitivity, protocols should include immediate availability of resuscitation equipment (e.g. epinephrine, oxygen), staff trained in managing anaphylaxis, and clear documentation of previous infusion reactions.
Summary
Intravenous iron enables rapid and predictable repletion of iron stores when oral therapy is ineffective, poorly tolerated, or too slow to meet clinical needs. After infusion, iron–carbohydrate complexes are taken up by macrophages, where iron is stored as ferritin and released gradually to transferrin for erythropoiesis—explaining the transient ferritin spike observed post-infusion.
Across diverse clinical settings—chronic kidney disease, heart failure, cancer, pregnancy, and perioperative care—IV iron consistently improves iron indices and often quality-of-life measures, though the evidence base and magnitude of benefit vary by population.
Adverse reactions are uncommon and typically mild, most representing complement-activation–related pseudoallergy (CARPA) rather than true anaphylaxis. Severe hypersensitivity reactions are exceedingly rare. Only iron dextran and ferumoxytol carry FDA boxed warnings for potentially fatal reactions; other formulations—iron sucrose, ferric gluconate, ferric carboxymaltose, and ferric derisomaltose—are safer but still warrant vigilance and appropriate monitoring during administration.
In practice: Judicious selection of formulation, dose, and setting ensures safe, efficient, and individualized iron repletion. With proper monitoring and follow-up, IV iron remains one of the most effective and well-tolerated parenteral therapies in contemporary medicine.