Fluid shifts play a critical role in the body’s response to illness, injury, and homeostatic imbalance. Under normal conditions, fluid moves between the vascular, interstitial, and intracellular compartments in a tightly regulated manner, governed by hydrostatic and oncotic pressures across capillary membranes. After events such as hemorrhage, inflammation, or sepsis, these forces become disrupted, leading to shifts in fluid that can have significant clinical consequences. For example, in acute blood loss, the body rapidly draws fluid from the interstitial space into the vascular compartment to restore circulating volume, while in systemic inflammation, increased capillary permeability and damage to the endothelial glycocalyx promote leakage of plasma proteins and fluid into the interstitium, resulting in edema. Understanding the principles behind fluid shifts, both under physiologic and pathologic conditions, is essential to managing volume status, interpreting laboratory changes, and guiding therapeutic interventions in clinical practice.
By definition, hemorrhage represents a loss of blood volume. If the hemorrhage is not to prove lethal, this volume must be restored promptly. Because in man there is no mechanism for adding RBCs to the circulation through a reflex such as splenic contraction, the restitution of blood volume is essential for cardiovascular stabilization. In its absence, total peripheral resistance will remain elevated, and cardiac output will remain depressed… If fluid is to enter the plasma from an endogenous source, it must do so from the interstitium. Accordingly, the rate and extent of entry will be governed by Starling forces. Drucker et al, 1981
Mechanisms of Capillary Fluid Exchange
The classic Starling principle describes fluid movement across capillaries as a balance between hydrostatic pressure pushing fluid out and oncotic pressure pulling fluid in, with filtration at the arterial end and reabsorption at the venous end. However, the revised Michel–Weinbaum model incorporates the role of the endothelial glycocalyx and the protein-poor subglycocalyx space, showing that reabsorption rarely occurs under normal conditions. Instead, most filtered fluid is returned via the lymphatic system. This modern understanding better explains phenomena such as edema in inflammation and the limited effectiveness of colloid infusions in restoring intravascular volume.
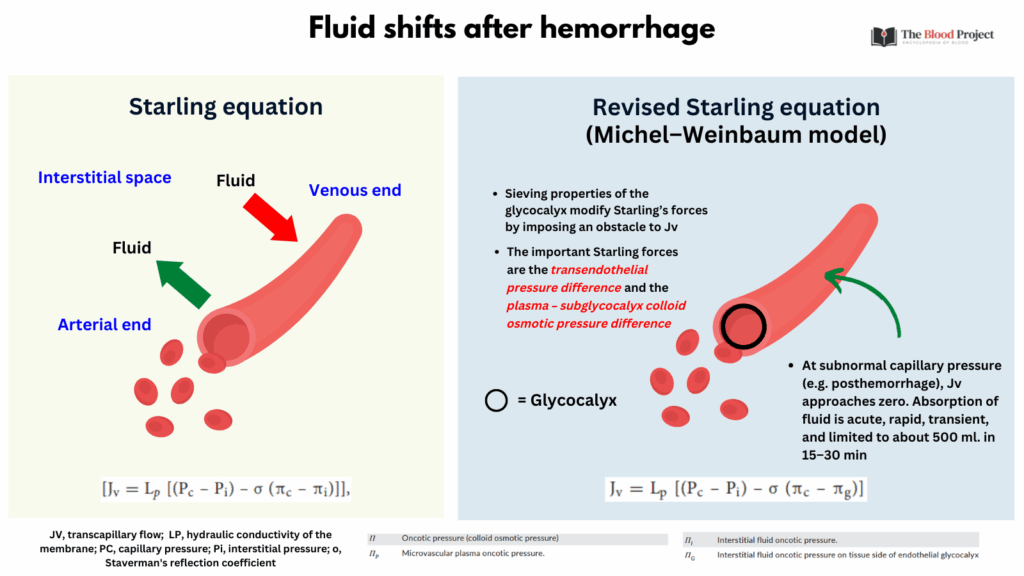
Starling Principle (Classic Model)
- Proposed by Ernest Starling in the late 19th century.
- Key Concept:
- Fluid movement across capillaries is governed by the balance of:
- Hydrostatic pressure (pushes fluid out)
- Oncotic (colloid osmotic) pressure (pulls fluid in)
- Fluid movement across capillaries is governed by the balance of:
- Starling Equation: Jv=Kf[(Pc−Pi)−σ(πc−πi)] where:
- Jv = net fluid movement
- Kf = filtration coefficient (capillary permeability)
- Pc = capillary hydrostatic pressure
- Pi = interstitial hydrostatic pressure
- πc = plasma oncotic pressure
- πi = interstitial oncotic pressure
- Classic View:
- Arterial end: net filtration (fluid exits capillary)
- Venous end: net reabsorption (fluid re-enters capillary)
Michel–Weinbaum Model (Revised/Modern View)
- Built on experimental advances in microvascular biology over the last ~30 years.
- Key insights:
- The endothelial glycocalyx (a carbohydrate-rich mesh on the inner capillary wall) plays a crucial regulatory role.
- Most protein filtration is blocked by this layer, altering the osmotic gradients.
- The sub-glycocalyx space has very low protein content, so effective oncotic pressure gradient is between plasma and this nearly protein-free layer, not the bulk interstitial fluid.
- Implications:
- Little or no reabsorption occurs at the venous end in most capillaries.
- Most filtered fluid is returned to circulation via lymphatics, not reabsorbed.
- Reabsorption only occurs in special circumstances (e.g., dehydration, hemorrhage, or certain tissues like the kidney).
- Supports why most capillary beds filter, and lymphatics, not veins, return that fluid.
Acute Hemorrhage
Acute hemorrhage: what happens initially
- Loss of blood volume:
- Hemorrhage results in loss of whole blood (both plasma and red cells), causing a drop in circulating volume, venous return, and cardiac output.
- Hemodynamic consequences:
- Blood pressure drops, especially systolic pressure.
- Reflex tachycardia and peripheral vasoconstriction (via sympathetic activation) attempt to maintain perfusion to vital organs.
Early fluid shifts (within minutes to hours)
- Fluid moves from the extravascular to the intravascular space after hemorrhage as part of the compensatory mechanisms to restore blood volume (autotransfusion of fluid from tissue to blood). The initial phase of this process, known as trans-capillary refill, involves the rapid shift of fluid from the interstitial (extravascular) space into the intravascular compartment.
- Fluid begins to move from the interstitial space into the circulation within minutes of acute hemorrhage, with the majority of the compensatory transcapillary refill occurring in the first 30–60 minutes.
- Experimental and human volunteer studies demonstrate that a substantial portion of the intravascular volume lost to hemorrhage is rapidly compensated by fluid recruitment from the interstitial compartment, with up to 25–50% of the lost volume replaced within the first hour, and the fastest phase of this shift occurring in the first 5–30 minutes.1
- Human volunteer studies indicate that after moderate blood loss, about 25% of the lost volume is compensated by fluid recruitment within 30 minutes.2
- The initial and clinically relevant phase of fluid recruitment is predominantly complete within the first hour after hemorrhage.
- Capillary refill from interstitium to plasma:
- Hydrostatic pressure in capillaries drops due to decreased blood volume.
- Oncotic pressure stays the same (plasma proteins aren’t lost in hemorrhage).
- This favors net reabsorption of interstitial fluid into the intravascular space.
- This shift can restore plasma volume by 500–1000 mL within 1–2 hours.
- Intracellular to extracellular fluid shift:
- Over time, water moves from the intracellular compartment to the extracellular space to support blood volume.
Hormonal responses that affect fluid balance
- RAAS Activation (Renin-Angiotensin-Aldosterone System)
- Promotes sodium and water retention by the kidneys.
- ADH (Vasopressin) Release
- Promotes water reabsorption in the kidneys.
- Also causes vasoconstriction to support blood pressure.
- Catecholamines
- Epinephrine/norepinephrine increase heart rate, contractility, and vasoconstriction.
Change in lab values
- Immediately after hemorrhage, hemoglobin and hematocrit may appear normal because plasma and RBCs are lost together.
- As fluid shifts into the vascular space, hemodilution occurs, unmasking anemia.
- Lactate and base deficit may rise, reflecting hypoperfusion.
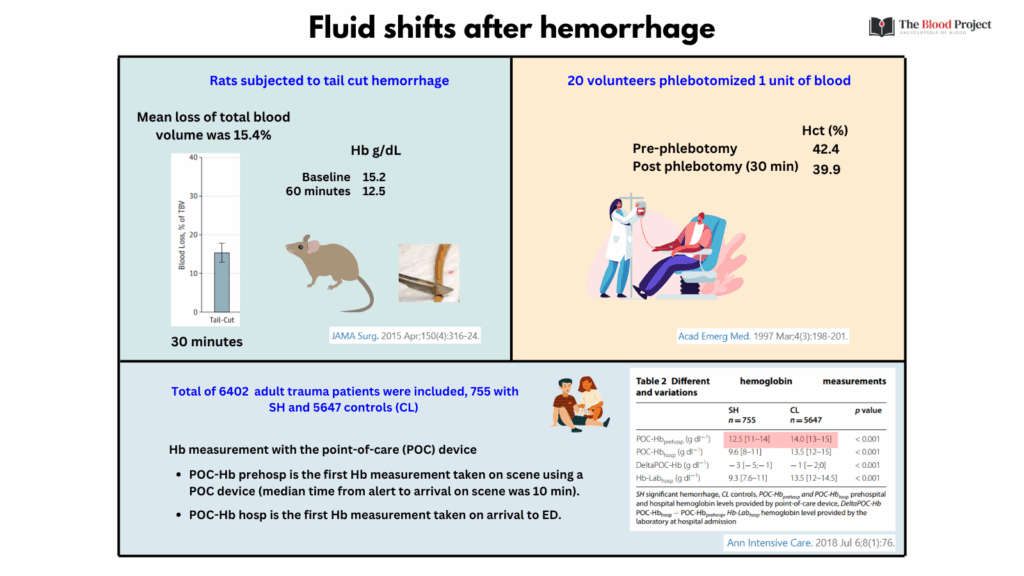
For larger image, click here.
Let’s consider the studies in the graphic above showing rapid drop in Hb/Hct following hemorrhage:
1. Morgan et al. Development and validation of 4 different rat models of uncontrolled hemorrhage. JAMA Surg. 2015;150:316-24
- Study Design & Methods:
- 4 different hemorrhage models using male Sprague-Dawley rats (6 rats/model), aged 10 to 14 weeks and weighing 330 to 460 g:
- Tail-cut (4 cm)
- Liver punch biopsy (12 mm)
- Liver laceration (3.0 × 1.5 cm)
- Spleen transection
- 4 different hemorrhage models using male Sprague-Dawley rats (6 rats/model), aged 10 to 14 weeks and weighing 330 to 460 g:
- Key Findings: (relevant to our discussion):
- The tail-cut model resulted in:
- Total blood loss 15.4% of total blood volume over 30 minutes, with most bleeding occurring within the first 2 minutes
- A significant drop in hemoglobin (2.7 g/dL [P = .004])
- The tail-cut model resulted in:
- Conclusion:
- The sharp reduction in hemoglobin signifies rapid intravascular volume loss, which triggers immediate fluid shifts from the interstitial and intracellular compartments into the vasculature.
- As blood leaves the vascular compartment, the body compensates via “transcapillary refill,” where fluid moves from the interstitial space into blood vessels. However, this shift dilutes the remaining red blood cells, contributing further to the observed acute drop in hemoglobin concentration after the initial hemorrhage.
2. Kass et al. Prospective crossover study of the effect of phlebotomy and intravenous crystalloid on hematocrit. Acta Emerg Med. 1997 Mar;4(3):198-201
- Study Design & Methods:
- A prospective, crossover volunteer study was performed comparing Hct changes immediately and 30 minutes after IV crystalloid bolus in 20 healthy adults with and without prebolus phlebotomy.
- In the control portion, volunteers were given a 15-mL/kg bolus of normal saline over 30 minutes with Hct determination before (Hl), immediately after (H2), and 30 minutes after (H3) crystalloid infusion.
- At least 7 days later, the same subjects were phlebotomized 1 unit of blood and then administered a 15-mL /kg IV bolus of normal saline 30 minutes later.
- Hcts were obtained before (H4) and 30 minutes after (H5) phlebotomy (immediately prior to crystalloid infusion). Hcts were also obtained immediately after (H6) and 30 minutes after (H7) crystalloid infusion.
- Key Findings: (relevant to our discussion):
- Phlebotomized individuals:
- 30 minutes following phlebotomy (but before crystalloid administration), the mean Hct dropped from 42.4 +/- 3.1 to 39.9 +/- 2.9 (H5).
- Phlebotomized individuals:
- Conclusions:
- “It has been well established that acute hemorrhage results in a decline in Hct due to movement of fluid from the extravascular to the intravascular space, otherwise known as “autoinfusion”. The concept of autoinfusion has important implications regarding the clinical assessment of blood loss.”
In the above study, phlebotomy (removal of one unit of blood) resulted in a decrease in Hct from 42.4 to 39.9% 30 minutes later. How much fluid shifted into the circulation?
Let’s walk through the calculation:
Assumptions:
- One unit of blood = 500 mL removed (mixed plasma + RBCs).
- The drop in hematocrit is not due to RBC loss alone, but also due to compensatory fluid shift into the intravascular space.
- No new RBC production during this short time frame.
- Initial blood volume ≈ 5 L (average adult).
Step 1: Estimate total red cell volume before and after:
- Let:
- Hctbefore=0.424
- Hctafter=0.399
- Vbeforeblood=5.0
- Vafterblood=5.0−0.5+fluid shifted
- We assume RBC volume lost = 0.424 × 0.5 L = 212 mL
- So original RBC volume = 0.424 × 5.0 L = 2.12 L
- After phlebotomy: RBC volume = 2.12 − 0.212 = 1.908 L
Step 2: Use hematocrit to solve for new total blood volume:
- Hctafter=0.399
- Vafterblood=1.908/0.399 = 4.782 L
- Thus, fluid shifted = Vafterblood−(5.0−0.5) = 4.782−4.5=0.282L=282mL
About 282 mL of fluid shifted from the interstitial to the intravascular space to partially compensate for the removal of one unit of blood.
3. Figueiredo et al. How useful are hemoglobin concentration and its variations to predict significant hemorrhage in the early phase of trauma? A multicentric cohort study. Ann. Intensive Care 2018;8:76
- Study Design & Methods:
- Large, multicenter retrospective cohort study of 6,402 adult trauma patients in six French trauma centers.
- Patients were included if they had a prehospital Hb measurement via a point-of-care (POC) device.
- Significant hemorrhage (SH) defined as receiving ≥4 units of RBCs in the first 6 hours or death from uncontrolled bleeding within 24 hours.
- Hb measurements were taken prehospital (POC-Hbprehosp), on hospital admission (POC-Hbhosp), and by laboratory (Hb-Labhosp). The difference between hospital and prehospital POC-Hb (DeltaPOC-Hb) was calculated.
- Key Findings: (relevant to our discussion):
- POC-Hbprehosp obtained on scene by the prehospital team (median time from alert to arrival on scene was 10 min) was significantly lower in patients who developed SH (median 12.5 g/dl) compared to patients who did not (CL; median 14 g/dl).
- Conclusion:
- Early, rapid drops in hemoglobin concentration can occur after trauma-related hemorrhage due to a combination of blood loss, early fluid resuscitation, and very early interstitial fluid shifts (refill).
- “The fair ability, albeit modest, of POC-Hbprehosp to predict SH challenges the conception that Hb and Hct in the early phase of hemorrhage are of little value because “the patient bleeds whole blood” and compensatory mechanisms for hypovolemia (transcapillary refill and fluid resuscitation) have not yet occurred.”
Key clinical implication
- After acute hemorrhage, the body rapidly pulls fluid from the interstitium to support circulating volume. This temporarily restores blood pressure but leads to dilutional anemia, which becomes apparent after several hours.
