Introduction
Have you ever asked yourself why we get sick? I don’t mean what body parts are responsible for disease, or what molecular pathways are involved. I mean why in a grander sense. Why, for example, are we so vulnerable to developing coronary artery disease and heart attacks, when so few other species on this planet suffer the same fate.1 Why do humans develop a dreaded disease like leukemia? Surely our bodies could (and, it may seem, should) have been more finely tuned.
The grander whys of medicine are the concern of an emerging field of medicine named evolutionary (or Darwinian) medicine. If you haven’t heard about it, you’re not alone. After all, it involves two unlikely bedfellows – evolutionary biology and medicine – and beyond a small, albeit growing circle of enthusiasts, it tends to be dismissed or outright ignored by the mainstream.
At first glance, these disciplines may seem fundamentally incompatible. Because evolutionary biology focuses on the deep past, and because it is population-based in its approach (how did humans evolve, rather than how did little Johnny develop a hare lip), it has little appeal to the physician treating Mr. Smith’s chest pain in the emergency room.
Conversely, evolutionary biologists are busy asking big questions about how species originated and how traits change over time scales that range over millions of years. With an orientation towards a macro perspective, it’s hardly surprising that many evolutionary biologists have largely steered clear of this hybrid field whose concern is human health. From within the silos of both evolutionary biology and mainstream medicine, the other can seem irrelevant in its disciplinary priorities and frameworks.
In spite of these apparent incompatibilities in orientation and scope, the fusion of evolutionary biology and medicine has revealed a gap in how we understand and approach disease, and it’s worth taking notice. The goals of this essay are to introduce the principles of evolutionary medicine, to draw comparisons between evolutionary and proximate mechanisms of disease, and to make a case for the incorporation of evolutionary principles in medical education, not as a revolutionary approach to improve patient care, but rather as a new mindset for understanding the human condition.
Evolutionary medicine as a field
Evolutionary medicine is the application of modern evolutionary theory to an understanding of human health and disease. Implicit in this definition is the application of certain core evolutionary principles that provide categories of explanation for complex genetic diseases. We will discuss each of these principles in turn, and then apply them to an understanding of blood disease.
On a personal note, I became convinced of the explanatory power of evolution after reading a book in 1996 titled Why We Get Sick: The New Science of Darwinian Medicine.2 With a stroke of the pen, the authors, a renowned evolutionary biologist, George Williams (1926-2010), and a clinician named Randolph Nesse (1948 – ), launched the field of evolutionary medicine, which has since grown to include multiple textbooks and journals, as well as a handful of departments and centers in American and European universities.

For example, The University of California, Los Angeles (UCLA) has started an Evolutionary Medicine Interdisciplinary Center, which provides coursework and educational opportunities to learn about evolutionary medicine at all educational levels. They also offer a master’s degree program in evolutionary medicine. Randy Nesse founded the Center for Evolution and Medicine at Arizona State University, a university-wide Presidential Initiative whose mission is to improve human health by establishing evolutionary biology as an essential basic science for medicine, worldwide.
Stephen Stearns, an evolutionary biologist and a member of the Advisory Board for The Blood Project, is a pioneer in the field of evolutionary medicine. He has published several books in the area and launched two peer-reviewed journals, Journal of Evolutionary Biology, and Evolution, Medicine, and Public Health.
Proximate and evolutionary mechanisms in health and disease
In their seminal book, Williams and Nesse draw on work by two evolutionary biologists, Ernst Mayr (1904-2005) and Niko Tinbergen (1907-1988), to highlight two different kinds of questions or explanations when approaching a biological entity, or trait.3
A trait can be anything from cell to complex organism, from animal behavior to human disease. The word signals that one is looking at something other than genes. From the perspective of blood, a red cell or the hemoglobin contained within the cell are examples of biological traits. So too are diseases such as sickle cell anemia and leukemia.
In 1961, Mayr suggested that biology should be approached from two complementary angles.4 Specifically, he distinguished between proximate and evolutionary questions. Proximate explanations describe traits and how they work. For most in the medical field, the proximal perspective represents the conceptual and cognitive framework for our models of learning, practice, and research.
By contrast, evolutionary questions ask why a biological trait is the way it is. When did it develop in evolutionary time, and what survival advantage does it provide at a population or species level? The distinction between proximate and evolutionary explanations is a core principle of evolutionary medicine and a critical theme in The Blood Project.
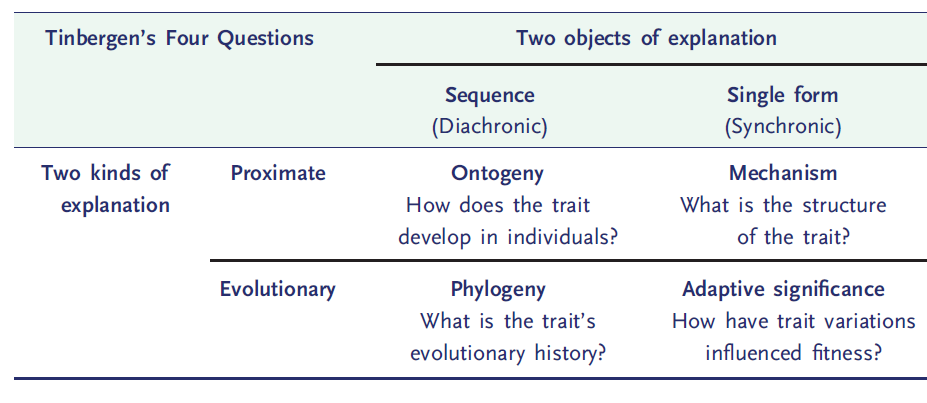
In 1963, Nobel laureate Nikolaas Tinbergen, a founding father of ethology, the study of animal behavior, famously proposed four major categories of explanations, now known as the “Tinbergen 4 questions”. The first two, mechanism and ontogeny, roughly map onto Mayr’s proximate mechanisms, while the second two, adaptive value and phylogeny, correspond to Mayr’s ultimate causes (Table 1). Tinbergen’s claim was that we cannot understand a biological structure or process until we have answered all four questions.
Let’s consider a familiar blood trait, the clotting system, through the lens of these 2 explanatory schemes. The clotting mechanism consists of a series of soluble proteins in the plasma portion of blood that act sequentially in a cascade of enzyme reactions, culminating in the formation of a glue-like fibrin clot. According to Mayr’s classification, we might say:
- The proximate cause of clotting involves disruption of the blood vessel wall leading to a cascade of reactions that ultimately yields fibrin clot.
- The evolutionary explanation is that blood clotting evolved as a mechanism to minimize the risk of fatal bleeding. Invertebrates, such as the crab or earthworm, have low pressure circulations that rely on blood cells called hemocytes to patch holes in the blood vessel wall. As animals evolved, their cardiovascular system acquired higher blood pressures to move blood around larger and more complex body structures and these high pressures, in turn, led to a greater risk of hemorrhage. The clotting cascade appeared for the first time in the ancestral vertebrate (which gave rise to all living vertebrates) around the same time that the blood became confined to the vasculature (a closed circulation) and blood pressures increased, some 450 million years ago.
Tinbergen’s four questions provide a slightly more granular approach:
- Mechanism (or causation), analogous to Mayr’s proximate mechanism, describes the physiology of the clotting system. What are the proteins, how are they activated, how do they interact with one another in the clotting cascade, and so on?
- Ontogeny describes the development of the clotting system over the lifetime of the organism, from fertilization to adulthood. It might address, for example, the observation that the clotting system becomes increasingly activated with age.
- Adaptive value (or function) describes how the clotting system contributes to reproductive fitness, arguing, as Mayr’s evolutionary mechanism does, that the clotting cascade improved reproductive fitness by providing a cover for high pressure cardiovascular systems.
- Phylogeny describes how the coagulation system has evolved over evolutionary time. For example, as discussed above, the clotting cascade as we know it is unique to vertebrates (fish, amphibians, reptiles, birds and mammals). It started out as an abbreviated system in fish (the first vertebrate class to appear), and became increasingly complex in amphibians, reptiles, birds, and mammals.
Some readers may be familiar with the proximate vs. evolutionary dichotomy. Others may find it perfectly intuitive. For some, these different levels of explanation will be completely novel. Regardless of where you fit on the knowledge spectrum, I would encourage you to consider the 2 x 2 box in the table above every time you consider a feature of the hematological system, whether in health or disease. Train yourselves to approach blood disease disorders as traits with both proximate and evolutionary explanations, and you will have discovered a whole new world of understanding.
Anemia of inflammation: a real-world example of proximate versus evolutionary considerations in medicine
Let’s consider an example in the field of hematology. Patients with chronic inflammatory disorders, for example rheumatoid arthritis or inflammatory bowel disease, often have a low hemoglobin, or anemia. This used to be called anemia of chronic disease, but is now more commonly referred to as anemia of inflammation. It is the most common cause of anemia in hospitalized patients and occurs not only in chronic inflammatory disease, but also in infections and malignancies. A remarkable feature of this type of anemia is that the hemoglobin rarely falls below a certain level (about 8 g/dL compared to a normal hemoglobin of 12-16 g/dL). It’s as thought there is an inbuilt floor or hemoglobin nadir.
For those of us who live and breath proximate mechanisms, we would describe how, in the setting of inflammation, certain mediators called cytokines promote the expression of hepcidin, a master regulator of iron metabolism. Hepcidin is a protein made by liver cells. Once it is secreted into the blood, it signals to cells in the gastrointestinal tract and macrophages (which are the major storage cell for iron) to shut down all iron trafficking. Think of a lockdown in a prison, when, with sirens blaring, a guard flips a switch that shuts all prison doors to prevent prisoners from exiting their cells. According to this metaphor, hepcidin is the throw switch, and the iron molecules are the prisoners locked in place. However, iron is required for erythroid development in the bone marrow. It is an essential nutrient for making hemoglobin in red cell precursors. The sudden, and sometimes profound, sequestration of iron inside macrophages results in starvation of erythroid progenitor cells and subsequent anemia. This situation has been called functional iron deficiency, that is iron-deficient erythropoiesis in the presence of abundant iron stores in the body.
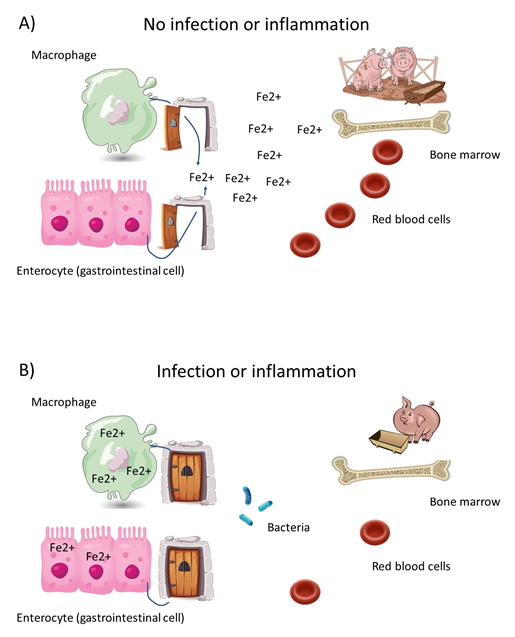
On the surface, we may conclude that anemia of inflammation is simply an adverse consequence of systemic illness. And what’s more, we may decide that treatment of the anemia is in order. Hemoglobin is a primary determinant of the oxygen carrying capacity of blood. Isn’t it wise to optimize a patient’s blood oxygen content, and hence oxygen delivery to the tissues? A consideration of the evolutionary implications of anemia of inflammation causes engenders a healthy dose of skepticism on this matter.
The sequestration of iron within macrophages, the hallmark of anemia of inflammation, has the potential benefit of depriving microorganisms of an essential nutrient. All organisms need iron to grow, some more than others. Perhaps, it has been argued, anemia of inflammation results from the body’s attempt to sequester iron from invading organisms. This would have been particularly adaptive in primitive man, when infection was the leading cause of mortality, and when humans did not live long enough to suffer significant chronic disease burden. According to this hypothesis, iron starvation of developing erythroid cells is a necessary trade-off (more on the concept of trade-offs below) in which the survival advantage associated with iron starvation of microorganisms outweighs the detrimental effect of low grade anemia.
But is it that simple? Rather than representing collateral damage, it is possible that the development of anemia is actually part and parcel of the adaptive response? Previous studies have shown acutely ill patients with anemia who are transfused to maintain a hemoglobin above 7-8 g/dL have the same or even better outcomes than those transfused with a hemoglobin less than 9-10 g/dL. Indeed, clinical practice guidelines recommend applying an Hb threshold of 7 g/dL in most patients.
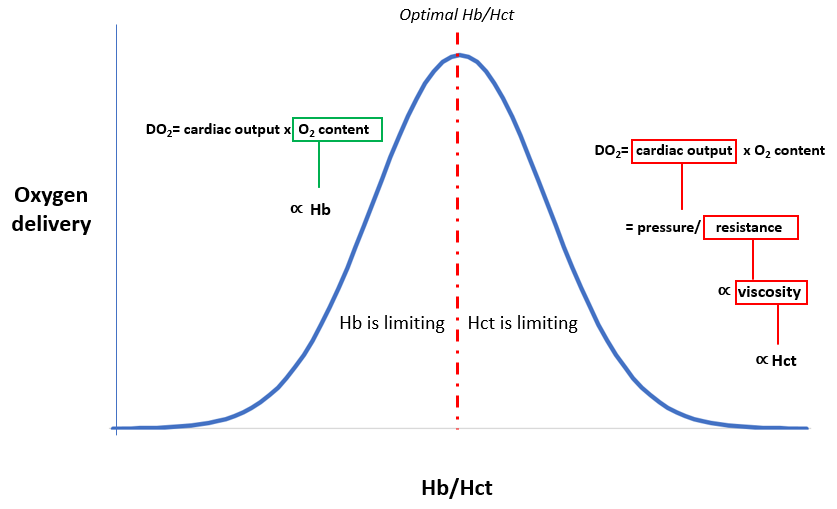
Is it just a coincidence that anemia of inflammation has a seemingly dialed-in hemoglobin nadir of about 8 g/dL, while empirical studies of transfusion triggers have arrived at the same ideal hemoglobin level? Maybe not. Hemoglobin levels are a two-edge sword. On one hand, the higher the hemoglobin, whose virtually sole function is to load, carry and unload oxygen, the higher the oxygen carrying capacity of blood. But, as we will discuss below, hemoglobin is packaged in red cells rather than being dissolved in plasma.
Red cells contribute to blood viscosity. In fact, the hematocrit (a measure of blood red cell number and volume) is the primary determinant of blood viscosity. As hemoglobin rises, so too does the hematocrit. There comes a point where the deleterious effects of the hematocrit on blood viscosity outweigh the beneficial effects of hemoglobin on oxygen carrying capacity of blood. This point is called the “optimal Hb/Hct”, a value that is highly conserved among mammals (about 15 g/dL/45%). In patients who are critically ill or inflamed, the optimal Hb/Hct may be shifted to the left (8 g/dL/24%). One reason for this shift is that non-cellular determinants of blood viscosity, especially fibrinogen and gammaglobulins, are increased as part of an “acute phase response”, in which the liver and white blood cells are repurposed to deliver disease-fighting substances. Perhaps the leftward shift in the optimal Hb/Hct serves to balance the increase in plasma viscosity.
Do these types of considerations have any importance in clinical medicine? If anemia of inflammation represents an evolutionary trade-off in which impaired erythropoiesis represents an undesired side effect of iron sequestration, then “fixing” the anemia, whether with red cell transfusions, oral or intravenous iron supplementation or erythropoietin administration (a hormone that promotes production of red cells) may be beneficial. However, if the anemia is part of the adaptive response, designed for example to offset the increased viscosity of plasma proteins, then perhaps it is best not to interfere. While there is no good answer as to whether inflammation of anemia is maladaptive or adaptive, these types of considerations open up a whole new level of thinking and discussion.
Core principles of evolutionary medicine
Evolutionary medicine is grounded in several core principles (Table 2). Among these are the optimization of reproductive success, the existence of evolutionary trade-offs, and a mismatch between genes and environment. We will discuss each in turn.
| Category | Core principle |
|---|---|
| Types of explanation | Both proximate (mechanistic) and ultimate (evolutionary) explanations are needed to provide a full biological understanding of traits, including those that increase vulnerability to disease |
| Reproductive success | Natural selection maximizes reproductive success, sometimes at the expense of health and longevity. |
| Trade-offs (evolutionary trade-offs) | Evolutionary changes in one trait that improve fitness can be linked to changes in other traits that decrease fitness. |
| Mismatch (reasons for vulnerability) | Disease risks can be altered for organisms living in environments that differ from those in which their ancestors evolved. |
| Defenses (reasons for vulnerability) | Many signs and symptoms of disease (e.g. fever) are useful defenses, which can be pathological if dysregulated. |
| Cultural practices (culture) | Cultural practices can influence the evolution of humans and other species (including pathogens), in ways that can affect health and disease (for example, antibiotic use, birth practices and diet). |
Reproductive success
Organisms are shaped by natural selection to maximize reproduction and secure the propagation of genes coded in the DNA. Evolution does not select for optimal health (as we define it), happiness, wealth or prosperity.5 Difficult as is it may be for us to accept, once we reach the age of 50 or so, we are largely expendable through the eyes of evolution.6 Now that we have spread our seeds (or missed our fleeting opportunity to so), our job is over and our future health is in the hands of the “gods” and our physicians. A humbling thought to be sure, but one that goes a long way towards understanding the origins of human suffering: chronic disease almost always strikes in post-reproductive years, and is therefore are not selected against. This leaves us to pick up the pieces, with the goal of abating morbidity and prolonging life.
There are many examples of hematological diseases that rear their ugly heads in the post-reproductive period. One such condition is a hematological malignancy called myelodysplastic syndrome (MDS). This disorder of the bone marrow slows down production of blood cells and may lead to debilitating infections (from low white blood cells), anemia (defined as low hemoglobin) and bleeding (from low platelets). The average age at diagnosis is about 75 years old, and only rarely do persons younger than 50 years old develop the syndrome. The bone marrow was never designed to survive into our 70s. At that stage of life, mutations in genes that affect blood cell growth are accumulating, probably at a rapid pace, and there is simply no incentive for natural selection to step in and construct a metabolically costly anti-mutation program, assuming that it could.
There is increasing evidence that genes conveying benefits at younger ages, for example those that promote juvenile survival or fertility, increase disease risk in old age. This trade-off – the beneficial action of genes at early stages of development, especially during an organism’s peak reproductive value, at the expense of deleterious effects in the post-reproductive period – is called antagonistic pleiotropy. Pleiotropy refers to the ability of a single genome (the genetic makeup of an individual) to confer more than one phenotype or trait, say at different stages of life. Their effects are said to be antagonistic because each gene has both beneficial and detrimental effects on fitness. Antagonistic pleiotropy, a popular explanation for the evolution of aging, helps to explain why many diseases have escaped the quality control of natural selection, and why certain disease-associated alleles are so prevalent in the population.
To return to our MDS example, a pervious genome-wide association study (an approach used in genetics research to associate specific genetic variations with particular diseases) of patients with MDS identified 8 discrete genes that were over-represented in this cohort.7 Notably, these genes have regulatory roles in the immune system, which are likely required for survival at a young age. In keeping with the theme of antagonistic pleiotropy, the results suggest that natural selection may have maintained genetic variation contributing to MDS because of its beneficial effects on fitness in early life. Another genetic study of patients with coronary artery disease provided compelling evidence that certain loci or genes associated with coronary artery disease later in life are enriched for fitness early in life.8
Evolutionary trade-offs
Another tenet of evolutionary medicine is that the human body comprises a set of design trade-offs. No trait is perfect, every trait may be made better, but improving one trait may make another one worse. Earlier, we discussed anemia of inflammation as a potential trade-off: iron sequestration in the name of reducing morbidity and mortality from infection at the cost of a lower hemoglobin.
In fact, the red cell provides a case study in trade-offs. Consider, for example, that the mammalian red cell is the only known eukaryotic cell type that lacks a nucleus.9 There are 10-15 million species of eukaryotes and many different cell types in multicellular organisms. If there was a ever a defining feature of a eukaryotic cell, it is the presence of a nucleus. What’s more, red cells survive without any cytoplasmic organelles, which are normally required to carry out basic cell functions, such as mitochondria, which consume oxygen to make energy, and ribosomes/endoplasmic reticulum, which synthesizes protein from messenger RNA.
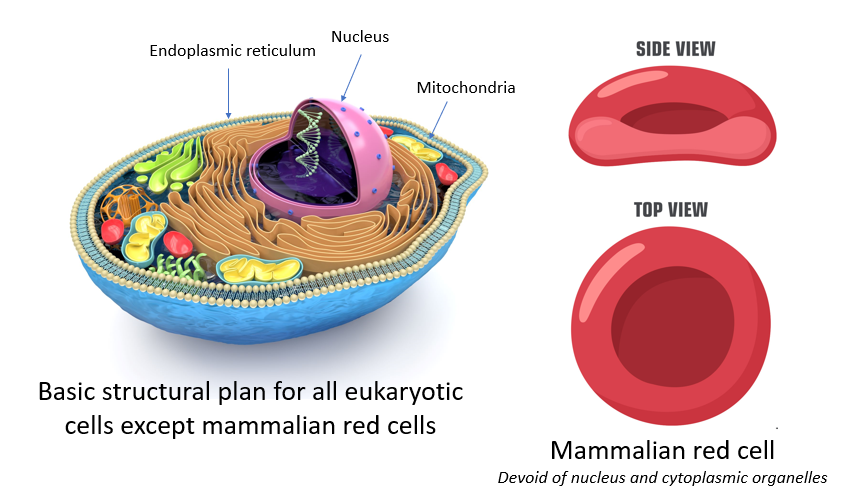
What is going on? Why is the mammalian red blood cell bucking the system? Why has it given up the very properties that define a eukaryotic cell? Let’s think this through: If you’re a red cell, one downside of losing your nucleus is that you can’t divide to make new cells. That puts more onus on the precursor cells in the bone marrow to maintain the red cell pool. If you lose your nucleus, along with ribosomes and endoplasmic reticulum, you can’t make new protein like other cells do. That limits your ability to repair yourself when you suffer wear and tear. It’s one reason why the lifespan of a human red blood cell is as short as it is (about 120 days). And if you have no mitochondria, you can’t make much energy, and energy is essential for fueling all the metabolic reactions that take place in the cell. These are some disadvantages of living without a nucleus.
But if the anucleate state were a detriment, it would have been selected against and quickly stamped out as soon as it appeared in the ancestral mammal some 150 million years ago.10 We need to come up with an answer as to why it was maintained in subsequent lineages. We can never know for sure (and this is one of the problems with evolutionary medicine – we can’t go back in time, often leaving us to speculate), but here are some thoughts: First, the nucleus is heavy. It’s like carrying a bowling ball around with you all the time. A typical human red cell weighs about 27 picograms. If we consider just the DNA from the nucleus of a human cell, that would add an additional 10 picograms to the erythrocyte, or roughly a 25% increase in weight.11 Because blood is in constant motion, weight counts for a lot. The heavier a cell, the more difficult it is for the heart to move blood around the circulation.
Second, the nucleus is relatively inflexible. That means when a red cell is squeezing through a tiny capillary, it would interfere with the cell’s ability to twist and turn. To accommodate their nucleus, animals with nucleated red cells (fish, amphibians, reptiles, and birds) are larger than their anucleate counterparts in mammals. That means the capillaries through which they traverse are wider. Larger capillaries mean less contact between red cells and the blood vessel wall, and lower capillary density in the tissues, with the net result being lower oxygen delivery to tissue cells.
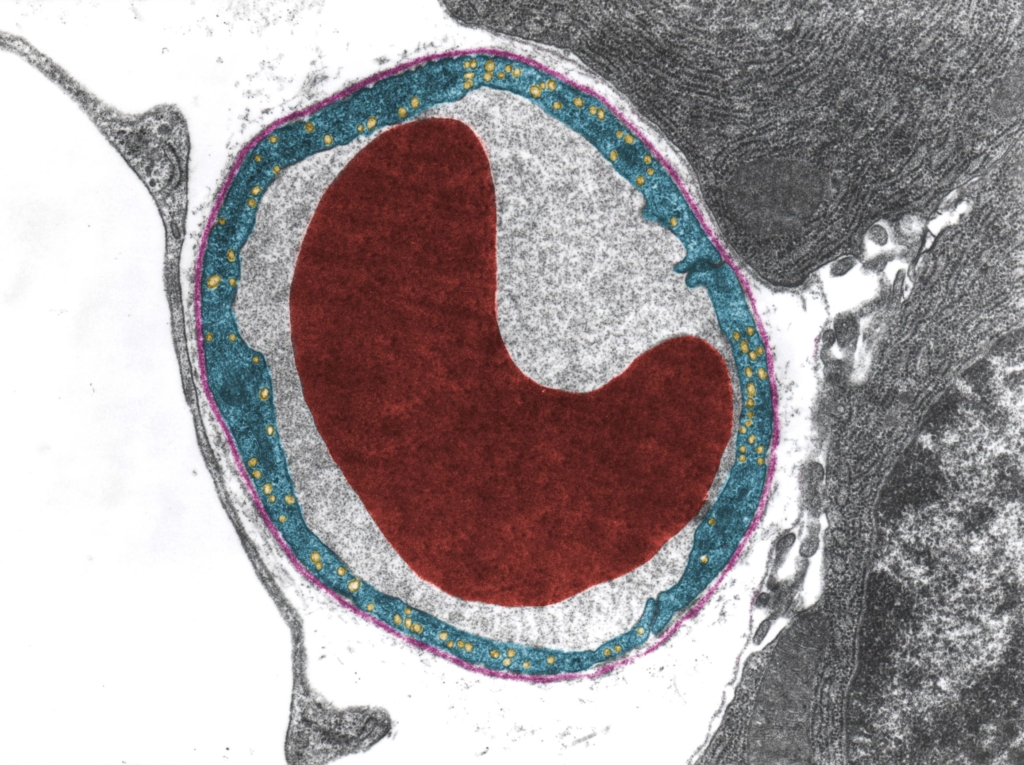
Third, the nucleus takes up precious room which could otherwise be occupied with hemoglobin, the protein that carries oxygen to tissues. A fourth argument in favor of ditching the nucleus is that red cells that lack mitochondria do not consume oxygen, and in this way, they avoid the “conflict of interest” of being both a consumer and carrier of the gas.
Regardless of the correct explanation, the loss of the red cell nucleus at the dawn of mammalian evolution is clearly an example of an evolutionary trade-off or design compromise, where the advantages of losing a nucleus (and other organelles) outweighed the disadvantages. We may not be able to use this information to inform our management of patients, but for those of who seek answers to “why” questions and not just “how”, it provides a powerful explanatory framework.
Another example of an evolutionary trade-off is the sickle cell trait. Hemoglobin contains a mixture of proteins called alpha and beta globins. Sickle cell trait (designated HbAS, with A referring to the wild type beta globin gene and S referring to the sickle allele) occurs when a person inherits a mutated beta globin gene from their mother or father. About 10% of African Americans have one copy of the sickle gene. This heterozygous genotype confers protection against severe malaria in malaria-endemic regions of the world, and that’s why it’s so prevalent. Because it provides a survival advantage, it has been selected for over the last 20-30,000 years. That’s the good news.
The bad news is that the protective effect of the HbAS genotype comes at a cost. When somebody inherits a sickle gene from each parent (now each of the two beta globin genes contains the mutation), they develop a serious, debilitating disease called sickle cell anemia (commonly designated as HbSS, with each S designating a sickle allele). Before modern medicine came up with stopgap measures to prolong the lifespan of such patients (or even cure the disease in the case of bone marrow transplantation), they rarely survived past age of 20 to 30 years.
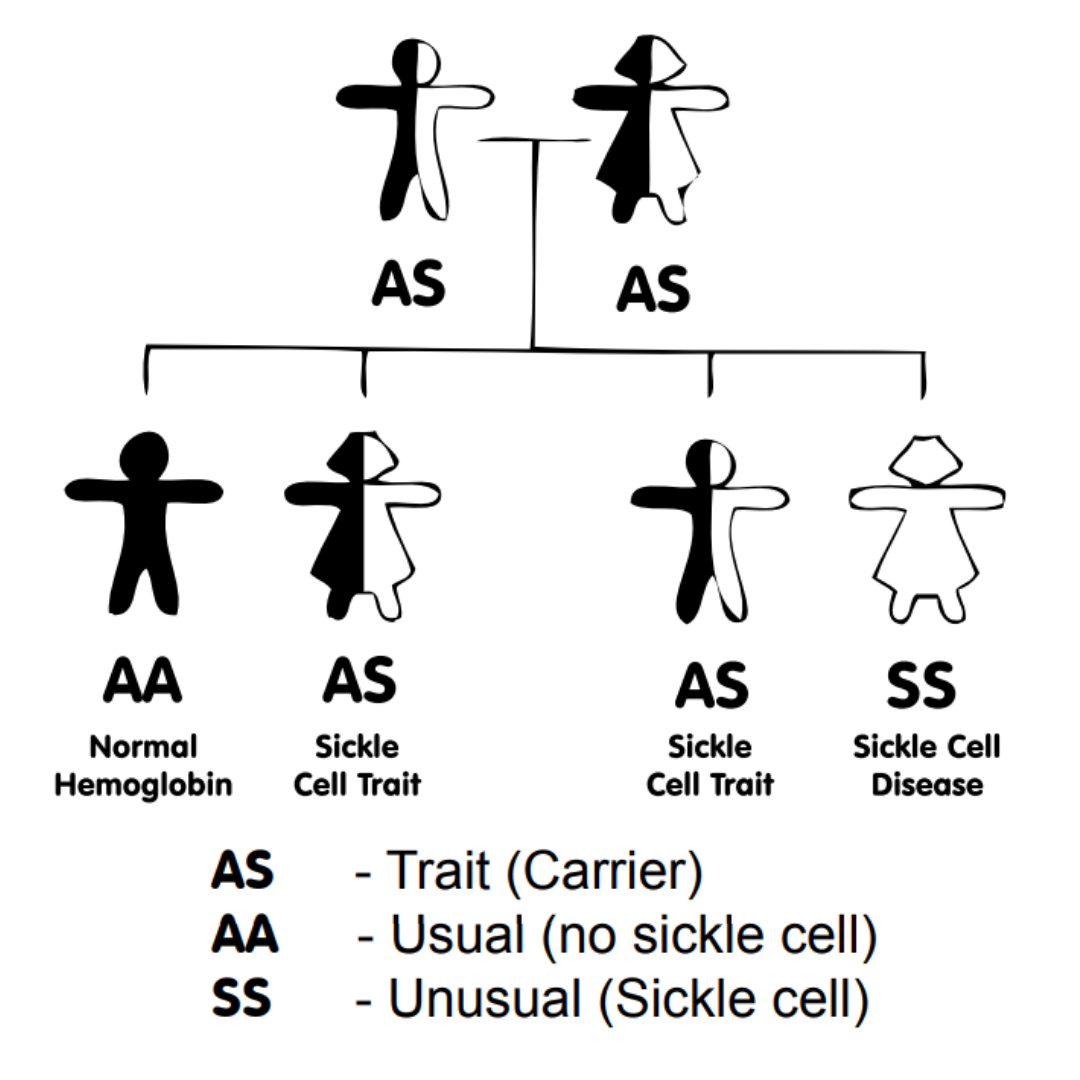
This situation is referred to as a balanced polymorphism, because individuals carrying a wild type (normal) copy of the gene (from one parent) and a mutated version of the gene (from the other parent) are better able to survive than those who have two copies of either version alone (two normal genes, or two sickle genes). The evolutionary process that maintains the two versions over time is called balancing selection. When viewed from an evolutionary perspective, all seems fair. But when seen through the lens of the individual with sickle cell disease, it’s another matter.
A third example of an evolutionary trade-off is the opposing effect of hemoglobin and hematocrit on oxygen delivery. As we discussed earlier, increased levels of hemoglobin improve the oxygen carrying capacity of blood, and thus oxygen delivery, while the associated rise in hematocrit increases blood viscosity, leading to reduced cardiac output and impaired oxygen delivery. If we plot Hb/Hct as a function of oxygen delivery, the curve is bell shaped with the apex representing the optimal hemoglobin/hematocrit.
One might ask: why package hemoglobin inside red cells (as all vertebrates do), if the cell mass only serves to increase blood viscosity and interfere with oxygen delivery? Why not dissolve the hemoglobin in plasma, like earthworms do?12
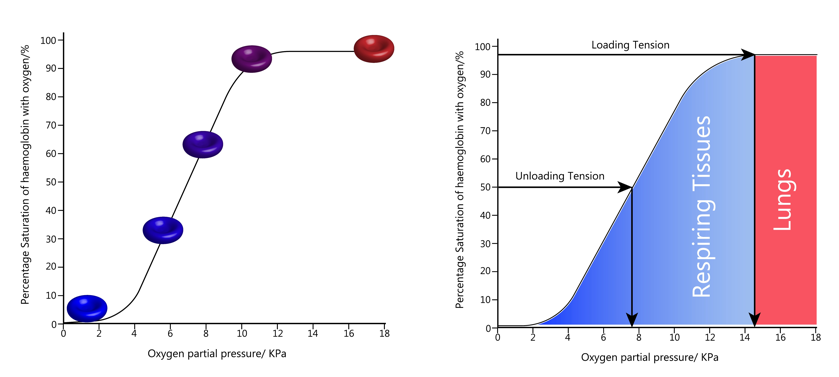
Hemoglobin exists as a tetramer of 4 subunits, which function cooperatively to meet the oxygen demands of the body tissues. Integrity of the tetramer structure is dependent on the red cell environment: when hemoglobin is released from red cells, for example in diseases associated with hemolysis, the tetramers are quickly degraded to dimers and monomers. Another function of the red cell is to prevent oxidation of hemoglobin, a deleterious chemical reaction that interferes with the ability to bind oxygen. Finally, the cytoplasmic environment of the red cell provides a means for fine-tuning the loading and unloading of oxygen.13 Collectively, these advantages of packaging hemoglobin inside red cells must have outweighed the disadvantage of red cells exerting an effect on blood viscosity and oxygen delivery.
It is perhaps no surprise that intensive research into hemoglobin-based oxygen carrier systems has largely failed to change transfusion practices. As a red cell surrogate, cell-free hemoglobin loses the protective environment of the erythrocyte and is rapidly degraded and oxidized. It also binds a gas called nitric oxide, which leads to vasoconstriction (narrowing of small arteries) and hypertension. To overcome these limitations, several modifications have been incorporated in the hemoglobin molecules, including cross-linking and polymerization to minimize dissociation of tetramers to monomers, and rapid clearance of hemoglobin. Despite such advances, research in this area has largely abated as attention has shifted to drug delivery platform technologies that encapsulate hemoglobin within cell-like microparticles and nanoparticles. This is a nice example of “Nature knows best”!
As a final example of trade-offs, consider the mountain climber who scales Mount Everest. The higher they climb, the less oxygen there is in the atmosphere. For example, at 12,000 feet, an altitude typical of the Tibetan Plateau, the oxygen concentration is only 60% of that available at sea level.14 As a result, oxygen content in the blood decreases and the body tries to compensate by increasing ventilation over the short term and increasing red blood cell production over the long term. The oxygen dissociation curve, which describes the binding of oxygen over a range of environmental oxygen pressures, shifts to the right. That means the efficiency of oxygen unloading from hemoglobin in the tissues is increased, while oxygen uptake by hemoglobin in the lungs is decreased.
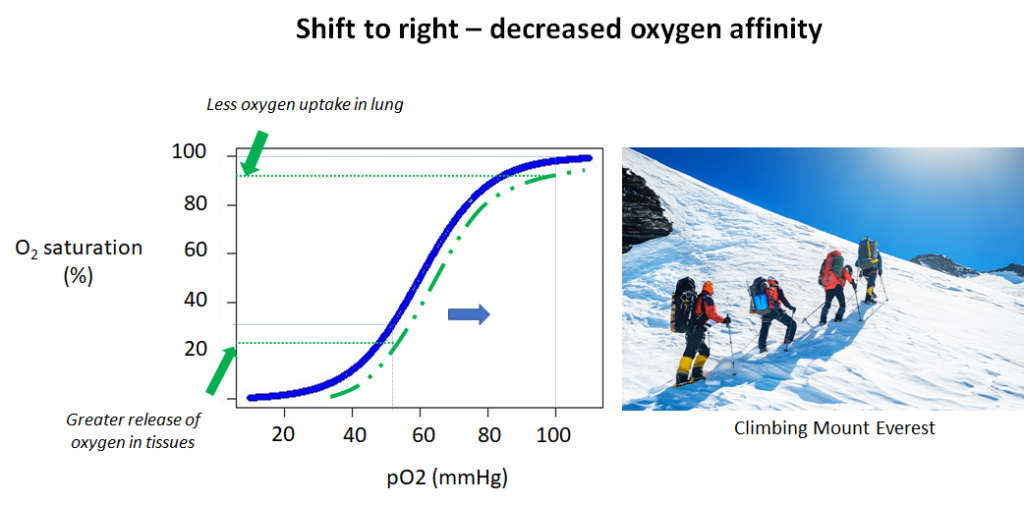
What would happen if the oxygen dissociation curve shifted in the opposite direction, namely to the left? In that case, oxygen loading in the lungs would increase, while unloading in the tissues would decrease. You will recognize the tradeoffs here. In one direction (shift to the right) you can optimize oxygen delivery at the expense of oxygen uptake in the lungs, while in the other direction (shift to the left), you may maximize oxygen uptake in the lungs while impairing unloading at the tissue level. Unfortunately, you cannot have it both ways: improved oxygen loading and unloading. That is a design constraint of the system.
So, is the human response to high altitude adaptive? How would we answer that question? For starters, we might look at organisms that, unlike our species, evolved at high altitude. And the data are unequivocal. Whether a bar-headed goose that migrates biannually over the Himalayan mountain range at altitudes of > 20,00 feet, or a llama, whose natural habitat is at > 12,000 feet above sea level, their oxygen dissociation curve is decidedly left-shifted. Their hemoglobin has a higher affinity for oxygen and therefore picks up more oxygen in lung. Such comparative analyses make a strong case for the adaptive value of a left shift in the oxygen dissociation curve.
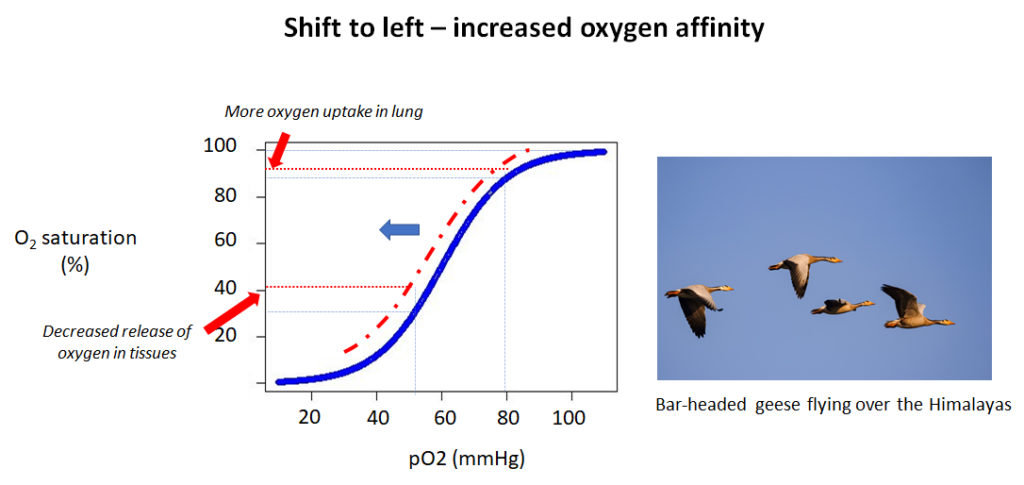
In a brilliantly creative experiment, Bob Hebbel, a hematologist at University of Minnesota, took 4 members of a family to about 10,000 feet in Colorado. Two of the family members had a congenital mutation in the beta chain of their hemoglobin that resulted in a shift of the oxygen dissociation curve to the left (a so-called high-affinity hemoglobin).15 The other 2 subjects were normal siblings. He showed that the high-affinity subjects, when compared with their wild-type siblings, fared better overall, including heart rate response and oxygen consumption. He concluded that “[the] results tend to contradict the belief that a decrease in hemoglobin oxygen affinity is of adaptive value to humans at moderate altitudes. Rather they support the hypothesis that, despite disadvantages at low altitude, a left-shifted oxyhemoglobin dissociation curve may confer a degree of preadaptation to altitude”. For those of us who would like to climb Mount Everest with the ease of a llama, perhaps if we took a page from nature and recalibrated our oxygen dissociation curve to the left, we would improve our performance capacity.

Humans have colonized multiple high-altitude locales, including the Tibetan Plateau, the Andean Altiplano, and the Semien Plateau of Ethiopia, for as long as 30,000-40,000 years. Studies of these populations provide interesting insights into the physiological and genetic adaptation of our species to high altitude. Interestingly, different high-altitude populations exhibit different adaptations. As one example, Andean highlanders have much higher hemoglobin concentrations compared to their Tibetan counterparts, living at similar heights. Though research to date has not uncovered a mutation in the hemoglobin gene that alters oxygen affinity, studies have revealed alterations in genes of the hypoxia-inducible factor (HIF) pathway, which orchestrates the transcriptional response to hypoxia under hypobaric conditions. These findings suggest that evolutionary forces have been at work for a relatively short period of time, and raise the possibility that a high affinity hemoglobin may someday appear in one or more of these mountainous populations.
Mismatch to modernity
Another core principle of evolutionary medicine is that there exists a mismatch between humans’ slowly evolving bodies and our rapidly changing modern environments. Our bodies were mostly selected for conditions that prevailed before improvements in agriculture, transportation. food distribution, industrialization, civic hygiene, clean water supplies, antibiotics, vaccines, and modern medical technology.16 Because biological evolution is slower than cultural change, some disease arises from the mismatch of our bodies to modern environments. As a greater proportion of the general population survives to older ages, the principle causes of mortality have shifted from the infectious diseases of childhood to chronic diseases associated with ageing.17.
Though not exactly a hematological disorder, the example of coronary artery disease that we considered at the beginning of the essay is particularly instructive in highlighting the gene-lifestyle mismatch hypothesis. Certain regions – or microdomains – of our coronary arteries, the major blood vessels that feed our heart, are particularly susceptible to atherosclerosis, the fatty plaque that builds up inside our arteries later in life.
In the paleolithic period, when we had a different diet, more exercise, and died younger for other reasons, the susceptibility of these arterial microdomains to atherosclerotic lesions was held in check and atherosclerosis was largely unheard of. Today, however, cultural changes, including smoking, inactivity and diet, have overwhelmed all checks and balances, and led to an epidemic in coronary artery disease. The gene-culture mismatch theory has been misappropriated by faddists pushing such trends as the Paleo diet. However, it has also been used more reasonably by others to emphasize the importance of good preventive medicine and public health.18
Conclusion
Evolutionary medicine has yet to realize its full potential. For one, it’s a hard sell for physicians, many of whom have been indoctrinated in proximate causation. Moreover, it hasn’t had a significant impact on patient care, other than to remind us of the importance of preventive medicine and to encourage us to consider that some symptoms, such as fever (and maybe anemia of inflammation), may represent an adaptation that perhaps be best left alone.19 Also, the field lacks a readily accessible research platform. Evolution operates over time scales longer than the lifetime of a patient. It’s one thing to speculate that the mammalian red cell is better off without a nucleus. It’s quite another to prove it.
For now, the value of evolutionary medicine lies is its explanatory power. For physician readers, the next time you peer down the lens of a microscope, ask yourself, “Why is the red cell shaped like a disc?” Or when you find anemia in someone with severe inflammation, consider not only the molecular mechanisms that caused the low hemoglobin or hematocrit in that patient, but also the evolutionary reason for this effect. The incorporation of evolutionary medicine may not impact your patients’ lives, but you will be a more thoughtful, creative and hopefully inspired physician for your efforts.
Acknowledgements
The author is grateful to Stephen Stearns for his invaluable input. The image of Charles Darwin at top of essay is from Vectorfarmer/Shutterstock.com.
About the author
William C. Aird received his MD from University of Western Ontario. He completed a fellowship in Hematology at the Brigham and Women’s Hospital, Harvard Medical School in Boston and is presently a practicing hematologist at the Beth Israel Deaconess Medical Center and Professor of Medicine at Harvard Medical School. He is founder and Executive Director of The Blood Project. Click here to learn more.
