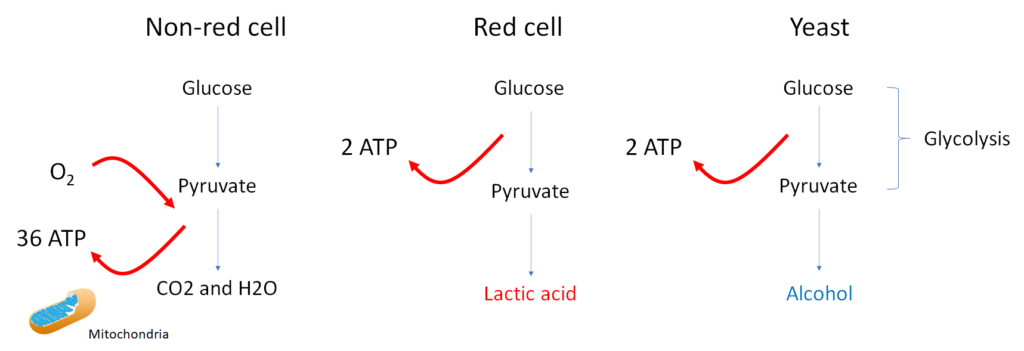
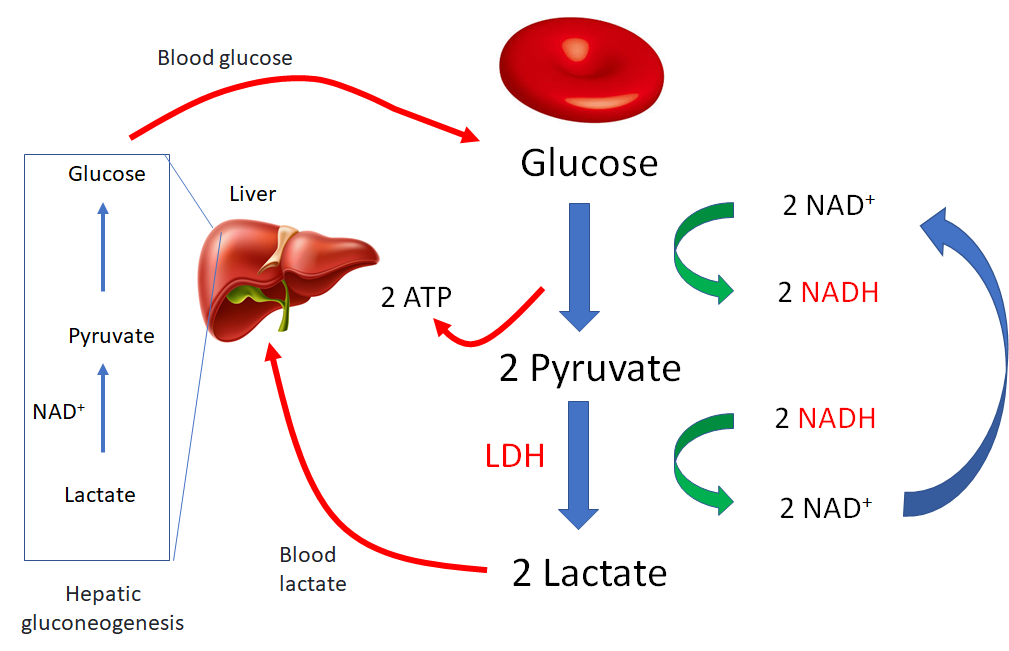
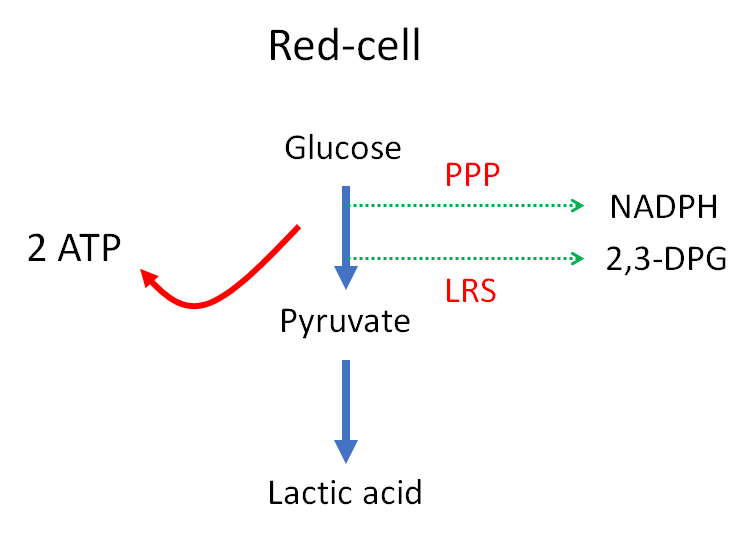
Did you know that red blood cells, like yeast that produce alcohol, are fermenters? In the presence of oxygen, other cells in the body break down glucose into carbon dioxide and water, and in the process generate some 36 molecules of adenosine triphosphate (ATP) for powering cellular activities, such as metabolic reactions, transport across membranes and mechanical work. Most of this energy is derived from the Krebs cycle and the electron transport chain, which operate within the confines of the mitochondria. When cells are deprived of oxygen (for example, ischemic muscle) or when they lack mitochondria (namely the mammalian erythrocyte), glucose is converted to lactate in a process called fermentation.1 Fermentation is an alternative non-oxygen-requiring pathway for generating ATP, though the yield of high energy phosphates is much less compared with aerobic respiration. This is an example of an evolutionary trade-off whereby the loss of mitochondria in the ancestral mammalian red cell provided a brilliant solution for transporting oxygen without consuming it, but at the cost of reduced ATP generation. To overcome this limitation, the red cell is an avid “eater” – it has the highest specific rate of glucose utilization of any cell in the body, approximately 10 g of glucose/kg of tissue/day, compared with ~2.5 g of glucose/kg of tissue/day for the whole body.2 The RBC uses most of this ATP to maintain electrochemical and ion gradients across its plasma membrane (these and other energy requiring functions are listed below). The lactic acid that is formed by red cells is released into the circulation, where it is taken up by liver hepatocytes. Hepatocytes then convert the lactate back to glucose, completing a cycle (glucose-lactate-glucose) that is referred to as the Cori cycle. The liver-derived glucose is then released for consumption by other tissues. When viewed from this perspective, the red cells help to feed the rest of the body!
Energy needs of red cell (learn more here):
- Maintenance of glycolysis
- Maintenance of the electrolyte gradient between plasma and red cell cytoplasm through the activity of adenosine triphosphate (ATP)-driven membrane pumps
- Synthesis of glutathione and other metabolites
- Purine and pyrimidine metabolism
- Maintenance of hemoglobin’s iron in its functional, reduced, ferrous state
- Protection of metabolic enzymes, hemoglobin, and membrane proteins from oxidative denaturation
- Preservation of membrane phospholipid asymmetry
| Term | Definition | Comments |
|---|---|---|
| Fermentation | Extraction of energy from carbohydrates in the absence of oxygen; end products include alcohol (yeast) and lactic acid (skeletal muscle, red cells). Produces 2 ATP molecules per molecule of glucose (from glycolysis, or conversion of glucose to pyruvate). | Occurs in some bacteria and yeast, in animal muscle during periods of intense exercise and in mammalian red cells. |
| Aerobic cellular respiration | During cellular respiration, a glucose molecule is gradually broken down into carbon dioxide and water in series of reactions that consume oxygen and generate energy in the form of high energy phosphates (ATP)P. About 36 ATPs are formed during aerobic respiration. | Requires the presence of oxygen and mitochondria. Red cells cannot respire because they do not have mitochondria. |
| Glycolysis | In glycolysis, glucose undergoes a series of chemical transformations, leading in the end to two molecules of pyruvate. Produces 2 ATP molecules per molecule of glucose. | Glycolysis itself does not require the presence of oxygen. It occurs as the first steps of glucose metabolism in both fermentation and aerobic respiration. The pyruvate generated by glycolysis either enters the Krebs cycle (aerobic respiration) or is converted to lactate or alcohol (fermentation). |