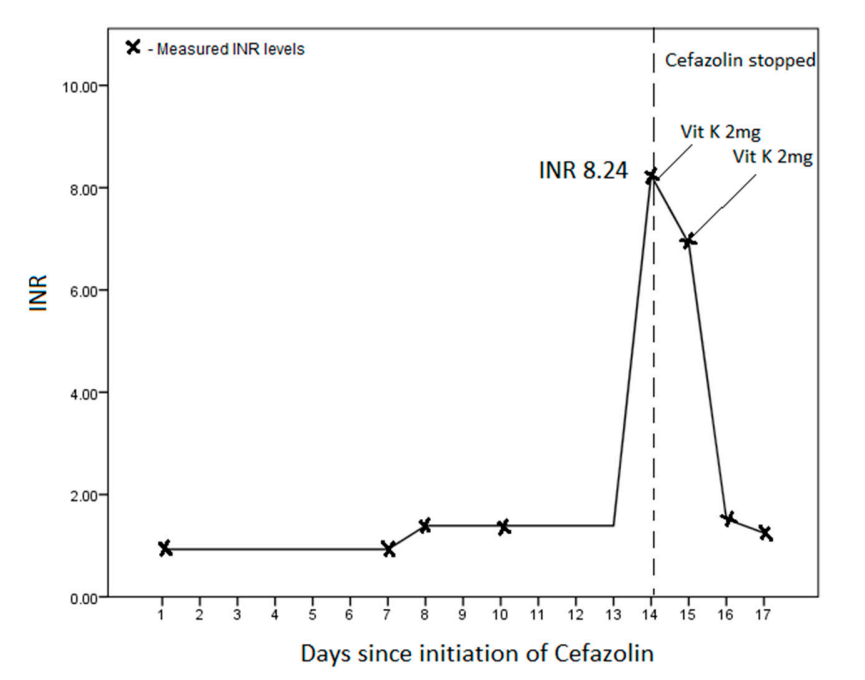
Case Study
- I posted the following data on Twitter and asked for a story:
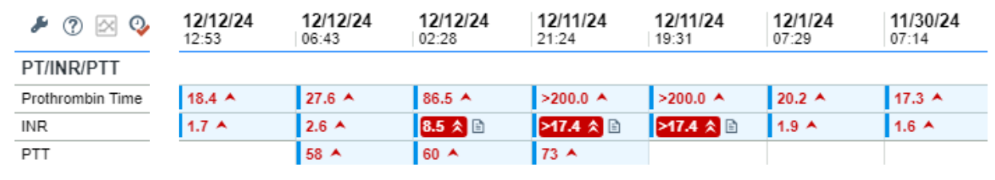
- Both the PT and aPTT are elevated. Thus, there is a deficiency of/inhibitor against factor(s) in the common pathway (X, V, prothrombin, fibrinogen) or combined factors in the intrinsic pathway and extrinsic pathway.
- There is an abrupt increase in the PT/INR arguing against a congenital factor deficiency or acquired inhibitor.
- The PT/INR is disproportionately affected relative to the aPTT, suggesting a preferential effect on FVII.
- This is a classic pattern for vitamin K deficiency, which results in reduced activity of FII, FVII, FIX, and FX. The effect on FVII is accentuated because FVII has the shortest half-life of the vitamin K-dependent factors.
- Malnutrition (without antibiotic use) does not cause acute vitamin K deficiency.
- Initiation of even high doses of coumadin would not increase the PT/INR this quickly.
- The cause in this case was the administration of IV cefazolin on 12/1/24 (the baseline INR of 1.9 was related to liver disease).
Introduction
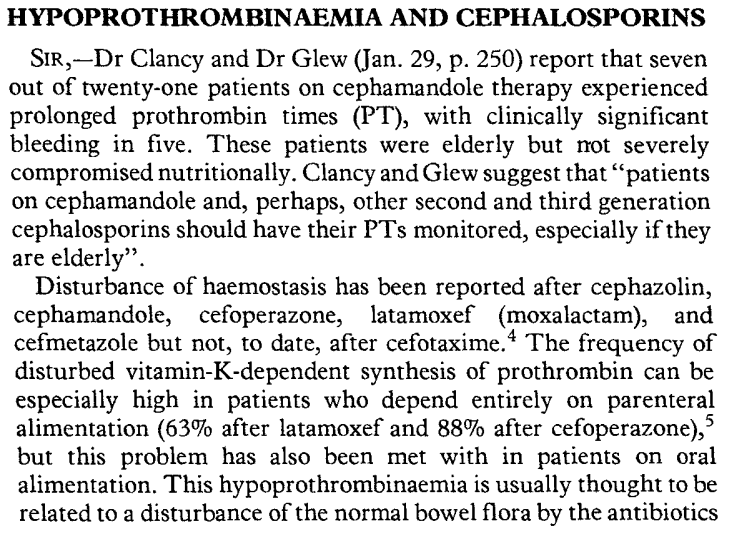
- Cephalosporins are commonly prescribed and widely used to prevent or treat various infectious diseases, including Gram-positive and -negative bacteria.
- Previous studies have shown a link between certain cephalosporins and coagulopathy.
- The association between NMTT-cephalosporins and hypoprothrombinemia was first reported in the 1980s.
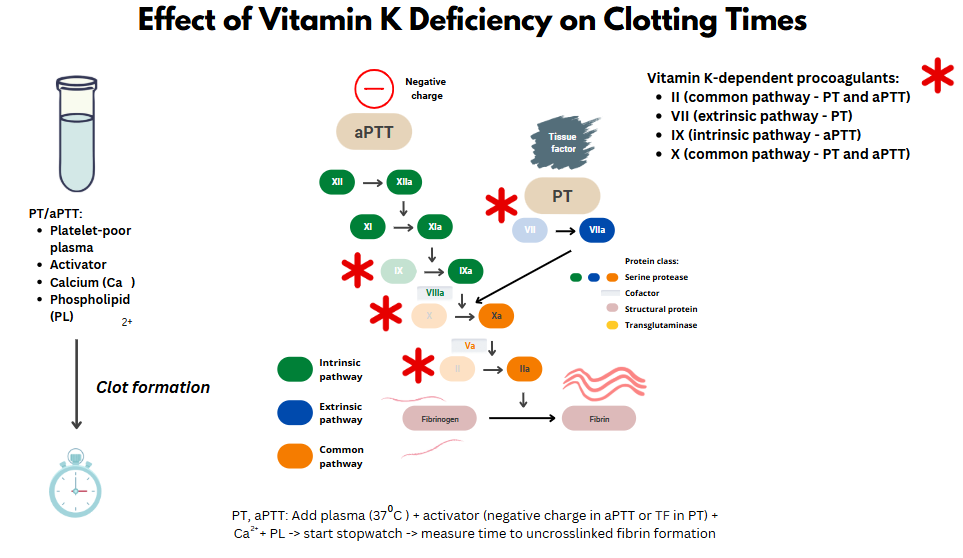
- Note: you will find the term hypoprothrombinemia in the literature relating to cephalosporin-induced coagulopathy. This is a misnomer. While the coagulopathy involves a reduction in prothrombin activity, it leads to lower activity of all vitamin K dependent factors (FV, VII, IX, X, and presumably PC and PS).
Mechanisms
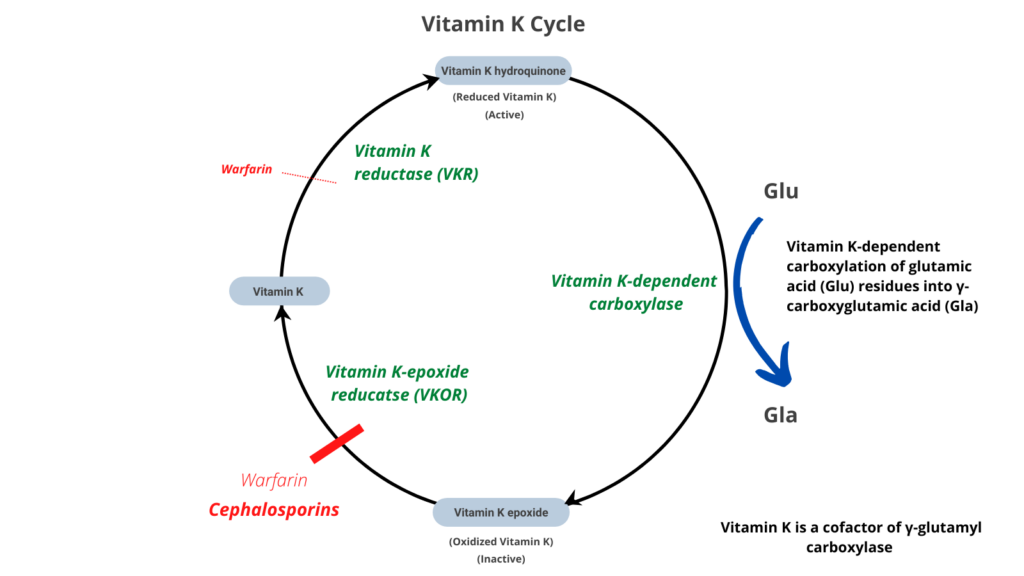
- Destruction of colonic microbiota, which produce the vitamin K (menaquinones [vitamins K2]) necessary for coagulation.
- Inhibition of vitamin K-epoxide reductase (and possibly vitamin K–dependent gamma-carboxylation of glutamic acid) by:
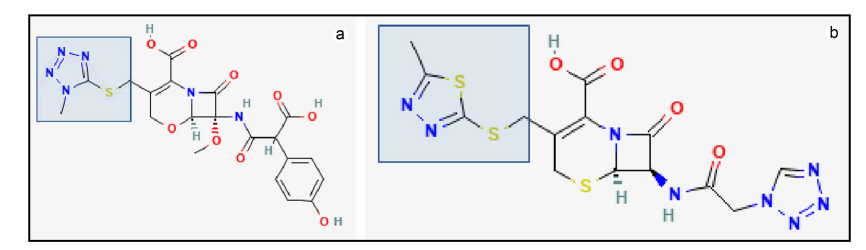
- 1-methyltetrazole-5-thiol or MTT side chain (moxalactam, cefamandole, cefotetan, cefmatazole and cefoperazone)
- 2-methyl-1,3,4-thiadiazole-5-thiol or MTD side chain (cefazolin, a widely used first-generation cephalosporin).
- MTT and MTD can undergo S-methylation by thiopurine methyltransferase (TPMT) to become less potent inhibitors of glutamic acid gamma-carboxylation. Thus, patients with loss-of-function mutations in TPMT who are treated with MTT- or MTD-containing cephalosporins may be more prone to coagulopathy.1
- Bleeding risk may be increased by:
- Poor nutritional status (due to reduced vitamin K intake).
- Renal disease (due to dissociation and accumulation of MTT or MTD side chains).
- Concomitant use of Rifampin.
Diagnosis
- Suspect the diagnosis in a patient receiving a cephalosporin who develops an elevated PT/INR level and a prolonged activated partial thromboplastin time (aPTT) while on therapy.
- These changes in PT/INR and aPTT occur as early as 2–4 days into therapy in some patients, and up to 14–21 days in others.2
Treatment
- Cessation of the cephalosporin
- Vitamin K supplementation
- Coagulopathy typically resolves within 48 h
Studies
- Chen et al:
- Nested case-control study within a cohort of 6191 patients who received hypoprothrombinemia-inducing cephalosporins and other antibiotics for more than 48 hours.
- 704 patients with hemorrhagic events.
- Use of hypoprothrombinemia-inducing cephalosporins was associated with increased risk of hemorrhagic events (aOR, 1.71; 95% CI, 1.42–2.06), which increased with higher cumulative doses.
- The adjusted odds ratios for individual cephalosporins:
- cefmetazole 2.88 (95% CI, 2.08–4.00)
- flomoxef 1.35 (1.09–1.67)
- cefoperazone 4.57 (2.63–7.95)
- Other risk factors included:
- Use of anticoagulants (aOR 2.08 [95% CI, 1.64–2.63])
- Liver failure (aOR 1.69 [1.30–2.18])
- Poor nutritional status (aOR 1.41 [1.15–1.73])
- History of hemorrhagic events (aOR 2.57 [1.94–3.41]) 6 months prior to the index date
- Authors’ conclusions: Use of hypoprothrombinemia-inducing cephalosporins increases risk of hemorrhagic events. Close watch for hemorrhagic events is recommended when prescribing these cephalosporins, especially in patients who are at higher risk.
- Park et al:
- Systematic review and meta-analysis were conducted to assess the safety profile of NMTT-cephalosporins with respect to hypoprothrombinemia and bleeding.
- A total of 15 studies on cefamandole, cefoperazone, cefotetan, cefmetazole, and moxalactam were identified and included in the meta-analysis.
- Hypoprothrombinemia (OR 1.676, 95% CI 1.275–2.203) and prothrombin time (PT) prolongation (OR 2.050, 95% CI 1.398–3.005) were significantly associated with NMTT-cephalosporins, whereas bleeding was not (OR 1.359, 95% CI 0.920–2.009).
- Subgroup analyses revealed that cefoperazone (OR 2.506, 95% CI 1.293–4.860), cefamandole (OR 3.247, 95% CI 1.083–9.733), and moxalactam (OR 3.367, 95% CI 1.725–6.572) were significantly associated with hypoprothrombinemia.3
- Authors conclusions: An Antimicrobial Stewardship Program led by a multidisciplinary team could play a critical role in monitoring cephalosporin-related hypoprothrombinemia or PT prolongation in patients with underlying clinical conditions at risk for bleeding. The multidisciplinary team could also assist in communicating the potential safety concerns regarding NMTT-cephalosporin use with healthcare professionals to decrease the risk of adverse events.
