Postscript
Introduction
- The thyroid gland plays an important role in body metabolism, including hematopoiesis.
- The American Thyroid Association estimates more than 12% of the US population will develop a thyroid condition and 20 million people have some form of thyroid disease.
- Abnormalities in thyroid function have been associated with several hematological abnormalities including:
- Anemia
- Alterations in the mean cell volume (MCV)
- Rarely pancytopenia (in myxedema coma)
- Changes in primary and secondary hemostasis
- Although the connection between thyroid function and hematopoiesis is not fully understood, evaluating thyroid function with TSH measurement is often recommended in the initial work-up of anemia or isolated macrocytosis.
Hypothyroidism and hyperthyroidism
- Hypothyroidism:
- Overt or clinical primary hypothyroidism is defined as thyroid-stimulating hormone (TSH) concentrations above the reference range and free thyroxine concentrations below the reference range.
- Subclinical hypothyroidism is defined by TSH concentrations above the reference range and free thyroxine concentrations within the normal range.
- The prevalence of overt hypothyroidism in the general population is 3–7% in the US.
- Hypothyroidism occurs more frequently in:
- Women
- Older people
- Patients with autoimmune diseases, including:
- Type 1 diabetes
- Autoimmune gastric atrophy
- Celiac disease
- Clinical manifestations of hypothyroidism range from asymptomatic to life threatening (myxedema coma).
- Symptoms may include:
- Tiredness
- Mental depression
- Sluggishness
- Cold intolerance
- Weight gain
- Dry skin and hair
- Constipation
- Menstrual irregularities in women
- Causes include:
- Primary (caused by destruction of the thyroid gland), most commonly:
- Autoimmune thyroiditis (Hashimoto’s thyroiditis).
- Severe iodine deficiency or excessive iodine consumption.
- Iatrogenic injury to the thyroid.
- Secondary (hypopituitarism, caused by TSH) deficiency), most commonly caused by compressive and/or invasive lesions affecting the pituitary sella region.
- Primary (caused by destruction of the thyroid gland), most commonly:
- Hyperthyroidism:
- Diagnosis of overt hyperthyroidism is based on suppressed or undetectable serum TSH and elevated serum T4 (either total [TT4] or free [FT4]) or T3 above reference ranges for thyroid hormones.
- Subclinical hyperthyroidism is low or undetectable serum levels of thyroid-stimulating hormone (TSH), but with free thyroxine (FT4) levels and total (TT3) or free tri-iodothyronine (FT3) within normal range.
- The prevalence of overt hypothyroidism in the general population is 1.3% in the US:
- 0.5% is clinical (overt)
- 0.7% subclinical
- Symptoms include:
- Heat intolerance
- Increased sweating
- Fatigue
- Nervousness
- Anxiety
- Poor concentration
- Insomnia
- Palpitations
- Shortness of breath
- Weight loss despite increased appetite (hyperphagia)
- Tremor
- Muscle weakness
- Oligomenorrhea or infertility in women
- Decreased libido in men
- Most commonly caused by:
- Graves disease
- Toxic multinodular goiter
- Toxic adenoma
- Painless thyroiditis
Anemia and changes in red blood cell volume
- Hypothyroidism
- Anemia has been reported in 20%-60% of hypothyroid patients.
- Thyroid diseases and anemia are common disorders and their prevalence increases with age:
- >10% of patients aged ≥65 years have anemia.
- 0.5% to 5% of patients aged ≥60-65 years have overt hypothyroidism.
- 5% to 20% of patients aged ≥60-65 years have subclinical hypothyroidism.
- Hypothyroidism is associated with macrocytosis:
- Macrocytosis reported in up to 35% of hypothyroid patients.
- Of those with macrocytosis, only 20% have anemia.
- Macrocytosis resolves with replacement therapy.
- Patient cohorts:
- Study of 8791 participants (European Prospective Investigation of Cancer (EPIC)-Norfolk population-based cohort):
- The mean age of the 8791 participants was 59.4 years and 55.2% were women.
- Thyroid dysfunction was present in 437 (5%).
- Anemia was present in 517 (5.9%):
- Among the anemic participants:
- 56 (10.8%) had iron deficiency.
- 58 (11.2%) had an inflammatory state.
- 6 (1.2%) had chronic kidney disease.
- 1 (0.2%) had an inflammatory state and chronic kidney disease.
- After excluding participants with these three most common causes of anaemia, the prevalence of thyroid dysfunction (abnormal TSH) was 12.6% (50 of 396 participants).
- Among the anemic participants:
- The prevalence of anaemia was:
- 4.5% among participants with euthyroidism.
- 7.7% in participants with overt hypothyroidism.
- 5.0% in those with subclinical hypothyroidism.
- 3.3% in those with subclinical hyperthyroidism.
- 14.6% in those with overt hyperthyroidism.
- Only 4.8% (19/396) of the anemic participants had overt thyroid dysfunction.
- Anemia in participants with thyroid dysfunction was:
- Normocytic in 94.0%
- Macrocytic in 6.0% (prevalence of isolated microcytosis [i.e., not associated with anemia] was not reported)
- Never microcytic
- Hb concentration was lower in participants with both hyper- or hypothyroid dysfunction compared to the euthyroid state; the prevalence of anemia was:
- Higher among adults with overt hyperthyroidism.
- Borderline significantly higher in those with overt hypothyroidism.
- Not different in those with subclinical thyroid dysfunction.
- The authors conclude:
- “The small prevalence of macrocytosis in our study is consistent with previous studies and further supports the fact that macrocytosis, although traditionally associated with hypothyroidism, is probably a rare finding in this population and rather relates to severe hypothyroidism.”
- “Although most guidelines recommend a systematic TSH measurement among patients with anaemia, screening for thyroid dysfunction leads to the identification of only 4.8% (19/396) of adults with overt thyroid dysfunction after excluding three other common causes of anaemia.”
- Study of 8791 participants (European Prospective Investigation of Cancer (EPIC)-Norfolk population-based cohort):
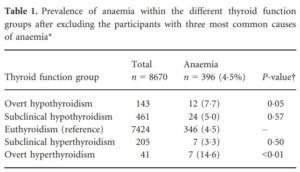
121 participants were excluded as they had iron deficiency (defined as ferritin <15 ng/ml, n = 56), inflammatory state (CRP ≥10 mg/l, n = 58), chronic kidney disease (estimated glomerular filtration rate ≤30 ml/min/1.73m2, n = 6), or both inflammatory state and chronic kidney disease (n = 1). In a sensitivity analysis using less conservative cut-offs for chronic kidney disease (eGFR <60 ml/min/1.73 m2), and iron deficiency (ferritin <30 ng/ml), 289/517 participants had at least one of these three common causes. Source.
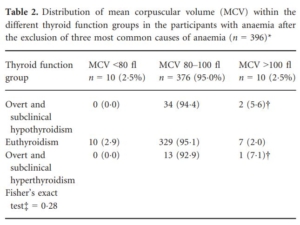
*defined as ferritin <15 ng/ml, CRP ≥10 mg/l and/or estimated glomerular filtration rate (eGFR) ≤30 ml/min/1.73 m2. †One participant with subclinical hypothyroidism, one participant with overt hypothyroidism and one participant with subclinical hyperthyroidism. ‡Fisher’s exact test for difference in MCV category (<80 fl, 80–100 fl, >100 fl) across the three different thyroid function groups (overt and subclinical hypothyroidism; euthyroidism; overt and subclinical hyperthyroidism. Source.
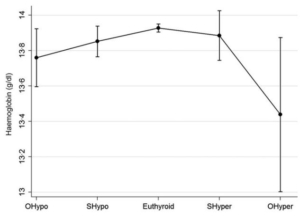
Comparison of hemoglobin (Hb) levels with 95% confidence intervals (CI) across thyroid function groups, adjusted for age and sex. Quadratic pattern linear analysis showed that participants in the euthyroid state had the highest levels of Hb, and that Hb values were lower in the participants with both hyper- or hypothyroid dysfunction, with a greater difference in the participants with overt than subclinical thyroid dysfunction (P-value for quadratic pattern <0.001). The only group which significantly differed from the euthyroid reference group was OHyper (Hb 0.49 g/dl), while the difference was borderline significant for OHypo (Hb 0.17 g/dl). OHypo: overt hypothyroidism; SHypo: subclinical hypothyroidism; SHyper: subclinical hyperthyroidism; OHyper: overt hyperthyroidism. Source.
-
-
- Study of 400 adults in ambulatory care:
- Prevalence of anaemia, after excluding other common etiologies, was significantly higher in patients with overt (43%) or subclinical (39%) hypothyroidism when compared with healthy controls (23%).[]Erdogan, M., Kosenli, A., Ganidagli[]
- Study of 42,162 adult participants:
- Of the 42,162 participants:
- 459 (1.1%) had overt hypothyroidism
- 2930 (6.9%) had subclinical hypothyroidism
- 36,081 (85.6%) were euthyroid
- 2386 (5.7%) had subclinical hyperthyroidism
- 306 (0.7%) had overt hyperthyroidism
- At baseline, 4274 (10.1%) participants had anemia:
- 15.9% in the overt hypothyroid group
- 11.6% in the subclinical hypothyroid group
- 9.7% in the euthyroid group
- 13.6% in the subclinical hyperthyroid group
- 11.1% in the overt hyperthyroid group
- Participants with subclinical or overt hypothyroidism and subclinical or overt hyperthyroidism had increased odds of having anemia compared with participants with euthyroidism.
- The pooled OR for the overt hypothyroid group was 1.84 (95% CI 1.35 to 2.50), 1.21 (1.02 to 1.43) for the group with subclinical hypothyroidism, 1.27 (1.03 to 1.57) for those with subclinical hyperthyroidism, and 1.69 (1.00 to 2.87) for those in the overt hyperthyroid group.
- Of the 42,162 participants:
- Study of patients aged 65 and older:
- Anemia was discovered in 155 of 316 (49%) enrolled:
- Causes included:
- Chronic disease (37.4%)
- Nutrient deficiencies (27.8%)
- myelodysplastic syndrome (5.2%)
- Other specific causes (14.8%)
- Unknown (14.8%)
- The prevalence of hypothyroidism was significantly higher in patients with anemia than in those without.
- TSH values were higher in participants with anemia (median 2.3 mU/L) than in those without (median 1.6 mU/L), whereas no significant differences in FT3 and FT4 values were reported between the groups.
- In subjects with hypothyroidism and anemia, TSH and hemoglobin levels were inversely correlated
- The authors state: “In 21 subjects with hypothyroidism and anemia, there were no other potential causes of anemia, underlining the importance of TSH levels to avoid an erroneous diagnosis of unexplained anemia.“
- Causes included:
- Anemia was discovered in 155 of 316 (49%) enrolled:
- Study of 202 patients with hypothyroidism:
-
-
- 172 women and 30 men.
- The mean hemoglobin concentration was 12.9 g/dl for both men and women.
- 53 patients with normal iron, B12 and folate; of these:
- 13/53 (26%) patients had anemia, which was presumed to be due to hypothyroidism.
- Average mean cell volume (MCV) in this group was 90 fL (range 83-113 fL).
- The MCV exceeded 90 fL in 29 of these 53 patients and in three (6%) it was greater than 100 fL.
- Nearly two-thirds of the anemic patients in this group had large red blood cells which diminished in size during treatment with thyroxine as the hemoglobin rose.
-
-
- Study of 400 adults in ambulatory care:
-
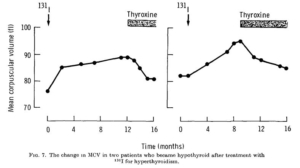
The change in MCV during the evolution of hypothyroidism in two patients who had been treated with radio-iodine for hyperthyroidism. Neither patient was anemic at any stage and both had normal blood levels of iron, folate and vitamin B12. In both the MCV rose progressively after radioiodine therapy and fell upon treatment of the supervening hypothyroidism.
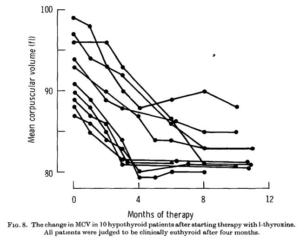
changes in MCV which occurred in 10 patients who were treated slightly differently but who were all judged to be euthyroid after three or four months. A sharp fall in MCV is evident in every case, even in those patients where it was initially within the normal range. The MCV became constant sooner after treatment in those patients whose average cell size was smaller to begin with. In such patients stabilization occurred at about the third month whereas in those patients with a marked degree of macrocytosis the period was much longer.
-
-
- Study of 782 outpatients with macrocytosis (MCV>99 fL):
- 1.2% had hypothyroidism (MCV ranged between 100 and 110 fL)
- 78% of these had anemia
- Study of 1,207 patients ag > 45 years
- 8.4% had MCV >99 fL
- Multivariate analysis showed strong and highly significant association with hypothyroidism (along with alcohol use, folate and B12 deficiency).
- Study of 782 outpatients with macrocytosis (MCV>99 fL):
-
- Hyperthyroidism
- Hyperthyroidism can result in changes in all blood cell lineages, including:
- Anemia
- Microcytosis
- Leukopenia (typically mild)
- Neutropenia (typically mild)
- Thrombocytopenia (typically mild)
- Pancytopenia (rare)
- Study of 200 patients with uncomplicated thyrotoxicosis:
- 37% had microcytosis.
- 8.5% had anemia.
- 11% had lymphocytosis.
- 2.5% had neutropenia.
- Study of 59 children with newly diagnosed Graves’ disease followed pre and post treatment with antithyroid drug therapy:
- Baseline results included:
- Decreased mean cell volume (MCV) in 32.2%
- Decreased Hb in 22%
- Deceased white cell count in 8.5%
- Deceased neutrophil count in 37%
- Thrombocytopenia in 5%
- Treatment for 4-6 weeks resulted in significant improvement in:
- Hb
- Platelet count
- Baseline results included:
- Study of 64 adults with Graves’ disease:
- 87 newly diagnosed patients with Graves’ disease.
- Twenty-one of 64 (33%) were anemic at presentation.
- Of these 21 subjects, a secondary cause of anemia could be established in 7 patients. Therefore, the prevalence of anemia without an obvious secondary cause (Graves’ disease anemia) was 14/64 (21.9%).
- The mean mean cell volume (MCV) was 80.3 fL ± 3.8, slightly below the lower limits of normal.
- Erythropoietin levels are inappropriately low for subjects with anemia.
- MCV did not differ between patients with Graves’ disease with and without anemia.
- After correction of hyperthyroidism, Hb levels improved in all but one patient.
- Hyperthyroidism can result in changes in all blood cell lineages, including:
Primary and secondary hemostasis
- Hypothyroidism is associated with:
- A shift of the hemostatic balance towards a hypocoagulable and hyperfibrinolytic (bleeding) state.
- Changes include:
- Acquired von Willebrand disease (VWD):
- Characterized by decreased concentrations of VWF and factor VIII (FVIII), and bleeding symptoms such as mild mucocutaneous bleeding and menorrhagia, but rarely major hemorrhage.
- Caused by a decrease of VWF protein synthesis in the absence of adequate levels of thyroxine (a synthesis defect with downregulation of VWF.
- Resolves completely after thyroid hormone therapy.
- Rare cases of non-immune thrombocytopenia.
- Decreased factor VIII (FVIII) levels.
- Hyperfibrinolysis (shorter clot lysis time).
- Bleeding tendency.
- Acquired von Willebrand disease (VWD):
- Hyperthyroidism is associated with:
- A shift of the hemostatic balance towards a hypercoagulable and hypofibrinolytic (clotting) state.
- Changes include:
- Increased activity levels of:
- vWF
- FVIII
- FIX
- Fibrinogen
- D-dimers
- PAI-1
- Decreased levels of:
- Plasminogen
- Tissue-type plasminogen activator
- Prolongation of clot lysis time and shortening of aPTT.
- Hyperthyroidism-induced vWF elevation is associated with enhanced platelet function and therefore shortened collagen-epinephrine-induced closure time (CEPI-CT) values.
- Thrombocytopenia:
- Usually immune thrombocytopenic purpura (ITP).
- Inhibited megakaryocytopoiesis is rarely described in severe hypothyroidism with bone marrow myxedema.
- Increased risk of venous thromboembolism (VTE).
- Increased risk of arterial thrombosis and mortality:
- Major embolism accounting for up to 18% of deaths in patients dying from thyrotoxicosis.
- The incidence of arterial thromboembolism in patients with atrial fibrillation is higher in those with hyperthyroidism than non-thyrotoxic individuals.
- Increased activity levels of:
- In a systematic review of hypothyroidism and acquired von Willebrand’s syndrome:
- One study was identified that reported 6.1% of 131 individuals with low VWF levels had a concomitant subclinical hypothyroidism as defined by normal thyroid hormone levels and raised thyrotropin (TSH) concentrations:
- Three of them (37.5%) had experienced bleeding symptoms.
- In all patients, normalization of hemostatic parameters was present after thyroid hormone replacement therapy.
- A total of 47 cases of acquired von Willebrand’s syndrome associated with hypothyroidism, both
overt and subclinical, have been described:- Mean age at diagnosis was 43.6 years and 30 of 43 patients were women (69.8%).
- Almost all bleeding episodes were mucocutaneous.
- Labs:
- von Willebrand factor antigen value:
- Available for 23 patients.
- Median value 28 U/dL (range: 4–45).
- VWF activity:
- Available for 24 patients.
- Median value 28.5 U/dL (range: <3–55).
- FVIII activity:
- Available for 16 patients.
- Median value 47 U/dL (range: 9–74).
- von Willebrand factor antigen value:
- One study was identified that reported 6.1% of 131 individuals with low VWF levels had a concomitant subclinical hypothyroidism as defined by normal thyroid hormone levels and raised thyrotropin (TSH) concentrations:
- Autoimmune thyroid disorders may be associated with:
- Immune thrombocytopenia
- Secondary antiphospholipid syndrome
- Acquired hemophilia
- Another systematic review led to the following conclusions:
- Thyroid dysfunction modifies the balance between coagulation and fibrinolysis.
- Coagulation tests revealed a hypocoagulable state for overt hypothyroidism and a hypercoagulable state for overt hyperthyroidism.
- The hypothesis of a hypercoagulable state in subclinical hypothyroidism is not supported.
- Few coagulation-fibrinolytic test abnormalities have been described in subclinical hyperthyroidism and
hypothyroidism.
Mechanisms
- Evidence for cause-effect relationship:
- Animal studies:
- Macrocytosis has been observed following thyroidectomy in animals.
- Hypophysectomized mammals found to have decreased red blood cell counts that correct following administration of thyroid hormones.
- Mice deficient in the thyroid hormone receptor have been found to have decreased hematocrit (detailed below).
- In wild-type mice, the clonogenic potential of progenitors in the erythrocytic lineage has been shown to be stimulated by thyroid hormone (T3), suggesting that T3 could directly accelerate the differentiation of progenitors to mature erythrocytes.
- Human studies:
- Congenital hypothyroidism (CH) has a high prevalence of anemia and immunodeficiency.
- Small case series in which treatment of hypothyroidism with levothyroxine resulted in a considerable increase in hemoglobin and resolution of anemia
- Patients with hyperthyroidism have a high prevalence of anemia, which returns to normal following antithyroid therapy.
- Animal studies:
- Physiology:
- Thyroid hormones modulate gene expression in virtually all tissues, orchestrating critical physiological processes for normal development, growth and energy metabolism.
- Thyroid hormone synthesis, secretion and action involve:
- Production of thyrotropin-releasing hormone (TRH) from the hypothalamus.
- TRH-mediated induction of thyroid stimulating hormone (TSH) secretion from the anterior pituitary gland.
- TSH-mediated stimulation of thyroid hormone production and secretion by the thyroid gland.
- Negative feedback of thyroid hormones on TSH release.
- Translocation of thyroid hormones across cell membranes via thyroid hormone transporters, where they bind to the nuclear thyroid hormone receptors (TRs), resulting in downstream changes in target gene transcription.
- There are 2 thyroid hormones:
- Triiodothyronine (T3) – mediates most actions of thyroid hormone.
- Thyroxine (T4) – a prohormone that is converted to T3 at the tissue level.
- Thyroid receptors (TRs)
- TRs are ligand-dependent transcription factors.
- There are two types (each with alternative promoters allowing the generation of several isoforms with different expression and functions):
- TRα (encoded by the gene THRA), including:
- TRα1 – essential for regulating erythropoiesis
- TRα2 – cannot bind hormone and may act as a TR antagonist.
- TRβ (encoded by the gene THRB), including:
- TRβ1
- TRβ2
- TRα (encoded by the gene THRA), including:
- TRα1, TRβ1 and TRβ2 can account for the majority if not all of TR-dependent thyroid hormone signaling.
- These receptors are comprised of modular functional domains that mediate the binding of hormones (ligands), DNA binding, receptor homo- and heterodimerization, and interaction with other transcription factors and cofactors.
- It has been demonstrated that TRs regulate the transcription of target genes by binding to specific DNA elements in the promoter regions of these genes, referred to as thyroid hormone response elements (TREs).
- Pathophysiology:
- Hematopoiesis:
- Hypothyroidism:
- In population-based cohort study:
- Free T4 but not thyroid stimulating hormone (TSH) was significantly associated with parameters of erythropoiesis, including hemoglobin concentration, hematocrit and erythrocyte count.
- There was no association between free T4 and mean cell volume (MCV).
- Patients with mutations of the TRα (THRA) have been reported to exhibit some of the classical symptoms and signs of hypothyroidism with:
- Impaired growth
- Delayed bone development
- Anemia
- Both juvenile and adult mice deficient for TRα have:
- Low hematocrit levels.
- Decrease in splenic numbers of B-lymphocytes, T-lymphocytes, macrophages and granulocytes.
- A mutant mouse expressing a mutated dominant negative form of TRα1 (denoted as TRα1PV/+ mouse):
- Reproduces the classical hypothyroidism seen in patients.
- Compared to wild-type mice, demonstrates decreased:
- Red blood cell (RBC) count (by 14%).
- Hb (by 13%).
- HCT (by 10%).
- Mean corpuscular hemoglobin concentration (MCHC) (by 3%).
- Platelet count (by 30% (panel F) lower in Thra1PV/+ mice than in WT mice.
- Erythroid lineage progenitors in the bone marrow.
- Terminal differentiation of progenitors in the erythroid lineage.
- Anemia is not a feature of patients or mice deficient in TRβ mutations.
- TRα1/TRβ KO female mice lacking the major receptors with the capacity to bind thyroid hormones vs. hypothyroid mice:
- Deletion of TRs cause a milder hematopoietic phenotype compared with hypothyroidism.
- Contrary to that observed in hypothyroid animals, TRα1/TRβ KO animals do not display overt anemia.
- The authors conclude: “In summary, our results show that mice lacking TRs do not display some of
the deleterious effects on erythropoiesis and leukopoiesis found in hypothyroid animals, indicating again the divergent consequences for hormone versus receptor deficiency… these results are compatible with a repressive action of the unoccupied receptors on hematopoietic gene transcription.”
- Pig model of hypothyroidism:
- Congenital hypothyroidism (CH) model in which homozygous missense mutation in the dual oxidase 2 (DUOX2) gene was generated by ethylnitrosourea (ENU) mutagenesis.
- Pigs displayed:
- Overt goiter.
- Significantly decreased thyroid hormone.
- Severe anemia with significantly lower levels of:
- Red blood cell count
- Hemoglobin
- Hematocrit
- T lymphopenia.
- Downregulation of the transcription factor, KLF9.
- Normal:
- White blood cell (WBC) count
- Mean corpuscular volume (MCV)
- Mean corpuscular hemoglobin concentration (MCHC)
- Overall, the findings support the critical role of TRα1 in erythropoiesis.
- In addition to the role of TRα1 in mediating erythropoiesis, other proposed mechanisms of anemia in hypothyroidism include:
- A direct β2-adrenergic receptor–mediated stimulation of red cell precursors.
- Thyroid hormone-mediated stimulation of erythropoietin production and/or release by the kidneys.
- Thyroid hormone affect on iron transport and utilization.
- Fe, as a constituent of thyroid peroxidase, is essential in the process of thyroid hormone synthesis.
- Studies in animals and humans have shown that iron deficiency anemia (IDA) impairs thyroid metabolism.
- Binding of thyroid stimulating hormone (TSH) to a functional TSH receptor on hematopoietic cells, which can be found in erythrocytes and some extrathyroidal tissues.
- T3-mediated augmentation of the release of growth factors from leukocytes that potentiate the erythroid burst-forming unit (BFU-E) proliferation.
- In hypothyroidism, normocytic anaemia may be a manifestation of a general decrease in basal metabolism
that results in reduced tissue oxygen demand. The pathogenesis of anemia in hypothyroidism may be
related to decreased oxygen requirement due to a decrease in
the basal metabolic rate - Relationship between hyperthyroidism and anaemia is less clear.
- Macrocytic anaemia in Hashimoto’s thyroiditis (HT) is frequently due to other autoimmune comorbidities, with pernicious anaemia being diagnosed in up to 10% of HT patients.
- CPG: While there is no consensus about population screening for
hypothyroidism there is compelling evidence to support case
finding for hypothyroidism in Those with pernicious anemia
- CPG: While there is no consensus about population screening for
- In population-based cohort study:
- Hyperthyroidism
- Mechanisms that increase erythropoiesis
- Thyroid hormone stimulates erythropoiesis leading to marrow erythroid hyperplasia and an
increased red cell mass via:- Direct effects on erythroid precursors.
- Indirect effects on erythroid precursors:
- Thyroid hormones may increase Epo levels:
- Directly
- Through increased oxygen demand
- T4 may also act directly on erythrocyte precursors through a receptor
with β2-adrenergic properties that can be blocked by β-blockers.
- Thyroid hormones may increase Epo levels:
- Thyroxine promotes heme synthesis and degradation in liver.
- Thyroxine shifts the Hgb dissociation curve to the right.
- Thyroid hormone stimulates erythropoiesis leading to marrow erythroid hyperplasia and an
- Mechanisms that decrease erythropoiesis:
- Blood volume is increased in hyperthyroidism by approximately 6%.
- Shortened erythrocyte survival.
- Ineffective iron utilization.
- Secondary causes including:
- Pernicious anemia
- Iron deficiency
- Mechanisms that increase erythropoiesis
- Hypothyroidism:
- Coagulation:
- von Willebrand factor:
- The molecular mechanisms of interaction between thyroid hormones, thyroid receptors and von Willebrand factor (vWF) have been investigated using in vitro studies; these showed that decreased vWF levels in hypothyroidism are mainly secondary to a reduction in synthesis and/or secretion, not to a reduced half-life due to autoantibodies or to molecular conformational changes.
- Fibrinogen and serine proteases:
- The liver:
- Is the primary site of synthesis of blood proteins involved in coagulation.
- Expresses high levels of thyroid receptors (TRs).
- Treatment of hepatocytes stably expressing TRα1 in vitro shown to:
- Increase expression of:
- Fibrinogen without requiring the synthesis of other proteins (i.e., direct regulation by T3 occurred).
- Prothrombin
- Factor X
- Tissue-type plasminogen activator (t-PA)
- Decrease expression of plasminogen.
- Increase expression of:
- The liver:
- von Willebrand factor:
- Hematopoiesis:
