How Does Ferritin Get Into and Out of Cells?
Prev
1 / 1 Next
Prev
1 / 1 Next
Though ferritin is a major intracellular storage depot for iron, it is present in small amounts in the serum. Serum ferritin is not simply a reflection of leakage from cells. Rather it is actively secreted by cells. Moreover, ferritin is taken up by cells, suggesting a potential cell-to-cell intercellular delivery mechanism for iron.
- Ferritin secretion by cells:
- Hepatocytes, macrophages and Kupffer cells have been shown to secrete ferritin, where it is found in the serum.
- Ferritin lacks a signal peptide sequence that is necessary for secretion through the classical (endoplasmic reticulum (ER)–Golgi) secretory pathway.1
- A variety of proteins have been shown to be secreted without passing through the organelles of the classical secretion pathway and are referred to as unconventionally (non-classically) secreted proteins. Such proteins are typically taken up into intraluminal vesicles of multivesicular bodies or autophagosomes and released upon fusion with the plasma membrane.
- Ferritin is one such example. It has been shown to be secreted through two nonclassical pathways:2
- A vesicular route that involves secretory lysosomes (i.e., trafficking via secretory lysosomes or secretory autophagy)
- An MVB–exosome pathway
- Thus, while ferroportin is the only known ferrous iron exporter, ferritin may function as an iron exporter of ferric iron.3
- Ferritin uptake by cells:
- Once released into the circulation, ferritin can be taken up by other cells, especially macrophages.
- Uptake may occur via:4
- Specfic receptors including:
- TIM2
- Human TfR1
- SCARA5
- Exosome-mediated transport
- Specfic receptors including:
- To explain why serum ferritin contains primarily L chains, it has been proposed that secreted ferritin with H chains are locally delivered to cells via vesicular structures, such as exosomes, and via receptor-mediated internalization and thus never reach the serum. “Only iron-poor L-subunit ferritins, which are barely taken up by the H-specific ferritin receptors, reach the serum”.5
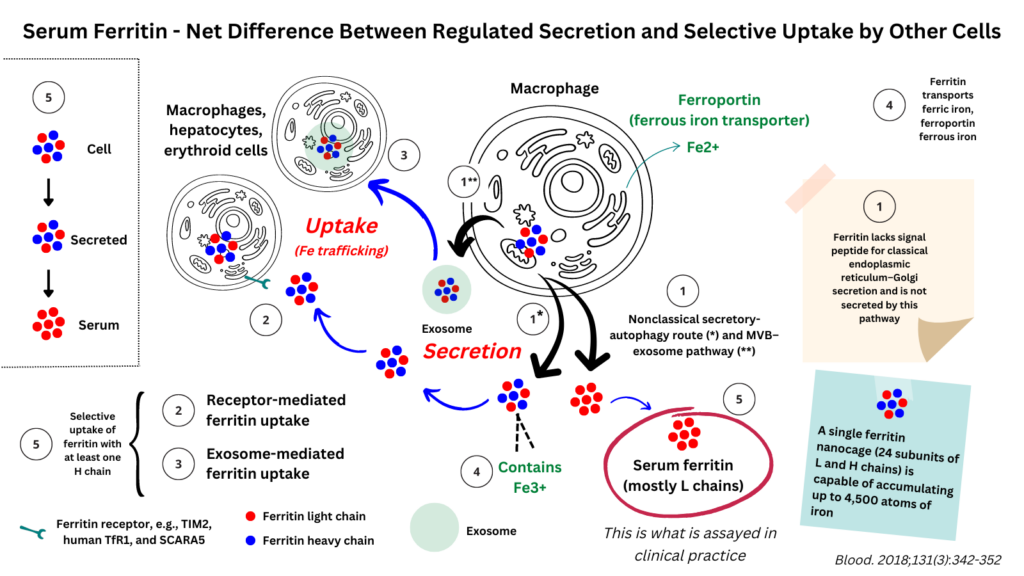
Figure key:
- 1) Ferritin is secreted by cells. It does not simply leak (though that may occur in cases of cell injury). Previous studies have shown that ferritin does not have a signal peptide for classical endoplasmic reticulum–Golgi secretion (though it does in insects). Rather, it is secreted by a nonclassical secretory-autophagy route (*) and MVB–exosome pathway (**).
- 2/3) Once secreted, ferritin is taken up by certain cells (e.g., macrophages, hepatocytes, and developing erythroid cells) by receptor-mediated and exosome-mediated mechanisms, where it provides a source of iron.
- 4) Thus, there are two ways to transport iron out of cells: ferroportin (Fe2+) and ferritin (Fe3+).
- 5) While serum ferritin is poor in H chains and Fe, secreted ferritin (e.g., from cultured cells) is similar to the intracellular form. To explain these differences, Truman-Rosentsvit et al have hypothesized that: “ferritins containing iron and at least 1 H subunit are locally delivered to cells via vesicular structures, such as exosomes, and via receptor-mediated internalization and thus never reach the serum. Only iron-poor L-subunit ferritins, which are barely taken up by the H-specific ferritin receptors, reach the serum.” In other words, what we are measuring clinically is the net difference between secreted ferritin (containing a wide spectrum of light and heavy chains) and what is selectively taken up by cells (primarily H chain-containing ferritin particles).6
Prev
1 / 1 Next
