Ferritin – Structure
Prev
1 / 1 Next
Prev
1 / 1 Next
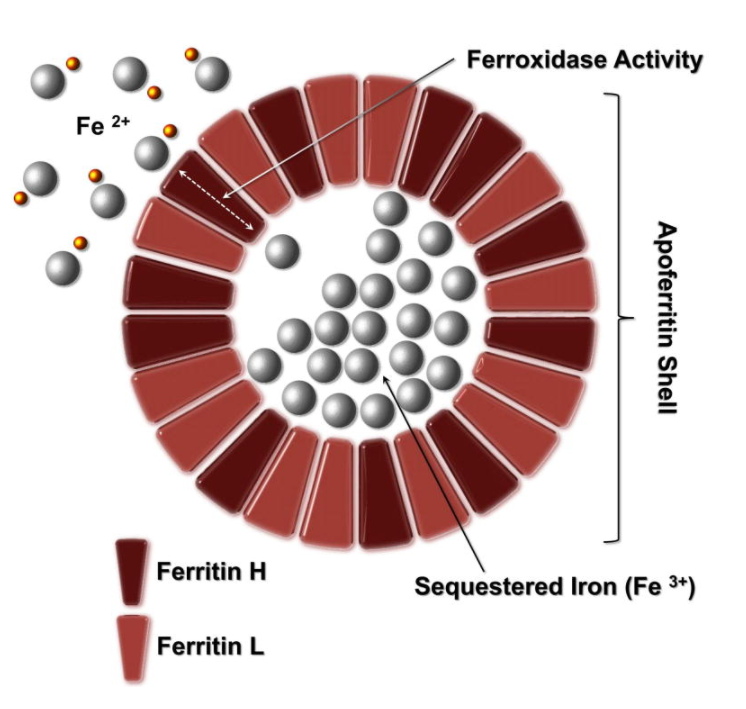
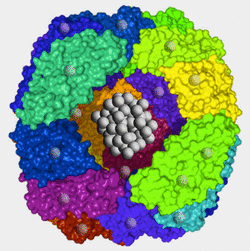
- Ferritin is a multisubunit protein with an outer protein shell and an inner cavity (protein cage) in which iron is stored in the Fe3+ form as ferrihydrite (inner mineral core).1
- The apoferritin shell has a molecular weight of about 450 kDa and a 7.0 nm-diameter internal cavity.2
- When fully saturated, a ferritin molecule can store up to 4500 atoms of iron, but the usual amount is closer to about 2000 atoms.3
- Ferritin is heterogeneous, differing between cell types and tissues in:
- Relative proportion of the different monomer subunits
- Iron content of its core
- Amount of carbohydrate
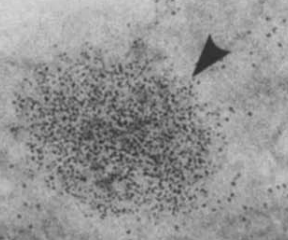
- By electron microscopy:4
- Iron-containing ferritin molecules are readily detected because of the electron density of the core.
- Typically, the particles have an octahedral appearance
- Ferritin molecules with maximal iron deposition in the core appear larger on electron micrographs
- Cytoplasmic ferritin may:
- Be randomly dispersed
- Occur in clusters
- Occur in lysosomes (termed siderosomes)
- Apoferritin refers to the iron-free form of the protein; the iron-containing form is termed holoferritin or simply ferritin.
Prev
1 / 1 Next