Ferritin Overview
Prev
1 / 1 Next
Prev
1 / 1 Next
Nature’s challenge:
- Too much iron leads to generation of reactive oxygen species and cell damage.
- Too little iron deprives cells of an essential nutrient.
Nature’s answer:
- Store/sequester iron in ferritin when iron levels are high.
- Release/recycle iron from ferritin when cell stores are low.
Overview
- When was ferritin discovered?
- Ferritin was discovered, crystallized and named in 1937 by the French scientist Victor Laufberger, who isolated a new protein from horse spleen that contained up to 23% by dry weight of iron.12
- In 1972, an immunoradiometric assay was developed and used to demonstrate that ferritin could be reliably detected in human serum.3. Serum ferritin was shown to be elevated in patients with iron overload and decreased in patients with iron deficiency diseases.4
- What is ferritin?
- Ferritin is an evolutionarily conserved globular 450-kDa iron storage protein (about 12 nm diameter overall) that consists of of an outer protein shell (2 nm thick) and a large hollow internal cavity (protein cage, about 8 nm in diameter) that can accumulate approximately 4,500 Fe3+ atoms in a mineral form (inner mineral core).5
- Ferritin is composed of 24 subunits (polypeptide chains) consisting of a mixture of heavy and light chains.67
- Ferritin heavy chain (FTH):
- The heavy chain monomer has 182 amino acids with a molecular weight of 21 kDa.
- The gene encoding the H subunit of human ferritin is located on chromosome 11q.
- FTH has ferroxidase activity that rapidly oxidizes Fe2+ into the ferric form (Fe3+) and incorporates iron into the ferritin mineral core.8
- At least one FTH subunit is required for iron loading into the ferritin shell.9
- The inactivation of FTH (but not FTL) is embryonically lethal.10
- Ferritin light chain (FTL):
- The light chain monomer contains 174 amino acids and has a molecular weight of 18.5 kDa.
- The gene encoding the L subunit of human ferritin is located on chromosome 19q.
- FTL does not have enzymatic activity. Rather, it promotes core nucleation and storage of Fe3+ into the ferritin core.
- While FTL stabilizes the iron core, FTL homopolymers are incapable of iron storage.11
- Serum ferritin is composed almost entirely of FTL.
- Ferritin produced by the lens of the eye consists entirely of L chains. This L chain ferritin is capable of forming crystals under certain conditions, as seen in the hereditary hyperferritinaemia cataract syndrome (HHCS).
- Amino acid sequence similarity between ferritin H and L subunits in mammals is about 50%.12
- FTH/FTL ratio:
- FTH and FTL subunits assemble in specific ratios to form the ferritin shell.
- Ratios differ:
by distinct genes located on human chromosomes 11 (for the H-chain) and chromosome 19 (for the L-chain) and have different promoters (description is from PMID: 37963965)
- Where is ferritin found?
- Ferritin is ubiquitously expressed in all tissues.
- It is found in particularly high concentrations at sites of high iron storage and recycling such as the bone marrow, spleen and the liver.
- Inside cells, ferritin is found primarily in the cytoplasm, but the H chain is also located in the nucleus, and a mitochondrial ferritin (FtMt) has recently been described.18
- Mitochondrial ferritin (FtMt) is an iron storage protein belonging to the ferritin family but, unlike the cytosolic ferritin, it has an iron-unrelated restricted tissue expression.
- In addition to its intracellular locations, small amounts of ferritin are found in the plasma (measured as serum ferritin).
- What is the function of ferritin?
- The main function of ferritin is to segregate inorganic iron in a non-toxic, protein-bound form while retaining high bioavailability.1920
- Other possible functions have been proposed including:23
- Intercellular exchange of iron via serum ferritin
- Regulation of angiogenesis
- Activation of pro-inflammatory signaling
- Prevention of vascular calcification
- Homeostatic regulation of heat and energy production
- What is ferritin a marker of?
- How does iron get into and out of the ferritin shell?
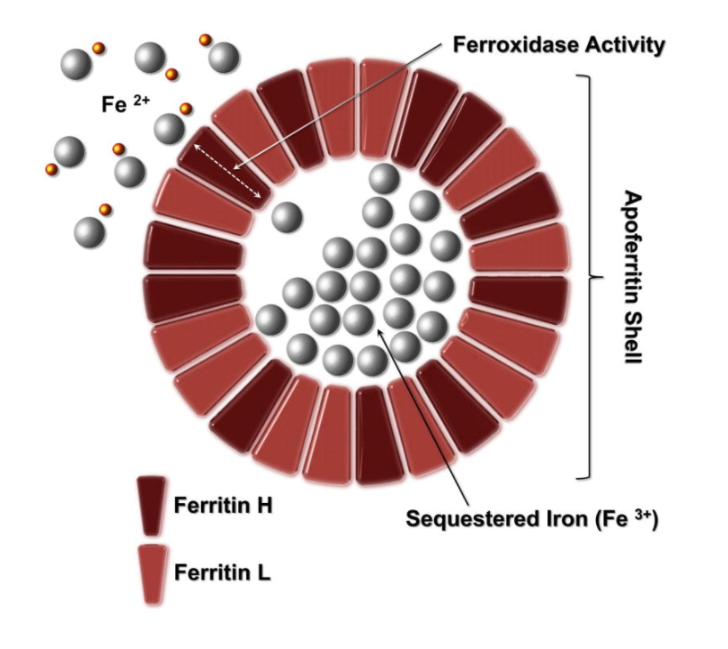
- Inorganic iron (Fe2+) is taken up through pores (iron channels) in the apoferritin shell.
- As the ferrous iron passes the pores, it is oxidized by the heavy chain (FTH) to Fe3+ and a growing crystal (FeOOH) of ferrihydrite is formed.26
- When iron levels in the cell are low, ferritin is degraded allowing the release of iron for use by the cell.
- Is ferritin evolutionarily conserved?
- Yes, it is present in most organisms, ranging from archeobacteria to mammals.
- Its structure is highly conserved, reflecting the important role of ferritin in iron homeostasis in species ranging from plants to humans.
Graphic Overview

Figure key:
- Ferritin heavy chain (FTH) (molecular weight of 21 kDa) is encoded by a gene located on chromosome 11q.
- Ferritin light chains (FTL) (molecular weight of 15.7 kDa) is encoded by a gene located on chromosome 19q.
- A total of 24 ferritin heavy and light chains assemble into a shell, creating a spherical protein that possesses a large cavity (protein cage). This iron-free protein is called apoferritin (molecular weight of about 450 kDa) (the iron-containing form is termed holoferritin or simply ferritin). The ratio of heavy to light chains is highly variable between cell types and tissues. The ratio is high in kidney, brain and heart, and low in liver and spleen.
- Ferrous iron (Fe2+) enters the apoferritin shell through iron channels or pores. FTH has ferroxidase activity that rapidly oxidizes Fe2+ into the ferric form (Fe3+).
- A small amount of ferritin is secreted into the extracellular space, and may be found in the serum. Serum ferritin is comprised almost exclusively of light chains and is iron poor.
- Ferritin expression is regulated by iron at a posttranscriptional level (both heavy and light chains) and by cytokines at a transcriptional level (primarily H chains).
- Ferritin is processed in lysosomes via several pathways:
- Secretion of ferritin into extracellular space and plasma (serum ferritin) via an NCOA4-independent mechanisms
- Iron release for use in the cell via NCOA4-dependent mechanisms
- Conversion/breakdown into hemosiderin, another storage form of intracellular iron.
Prev
1 / 1 Next
