Postscript
Context:
“Space exploration adds an entirely new dimension to biology. All bioorganisms that have evolved on the earth have done so under the influence of the gravitational force of this planet. They also live and function under this influence, which is so ubiquitous that is almost ignored. It is not known how this gravitational force, or its absence, may affect the many facets of life. The discipline of space biology must deal with this subject. Although the emergence of the space biology offers almost limitless opportunities for such studies, several concrete and potentially fruitful lines of inquiry appear to have already resulted from the orbital flights thus far conducted. One of these deals with the effects of gravity on bone formation, erythropoiesis, and the interrelation of these two processes. It has delineated several areas for study and has raised new questions in hematology. With the future launch of missions dedicated to biology, partial answers could improve our understanding of hemopoietic regulation.”
Blood.1982 Nov;60(5):1059-67.

Background:
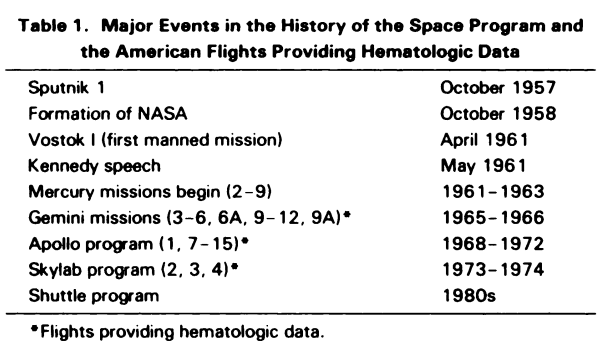
- October 4, 1957: the first artificial satellite, Sputnik I , was launched.
- July 29, 1958: NASA was formed.
- April 12, 1961: Yuri Gagarin was the first human in space (on Vostok 1).
- 1961: NASA mobilized industry, science, and technology, and began project Mercury.
- This was followed by Gemini missions and missions associated with the Apollo, Skylab and Shuttle programs, and more recently the International Space Station.
- As of 2019, 559 humans had been flown into space.
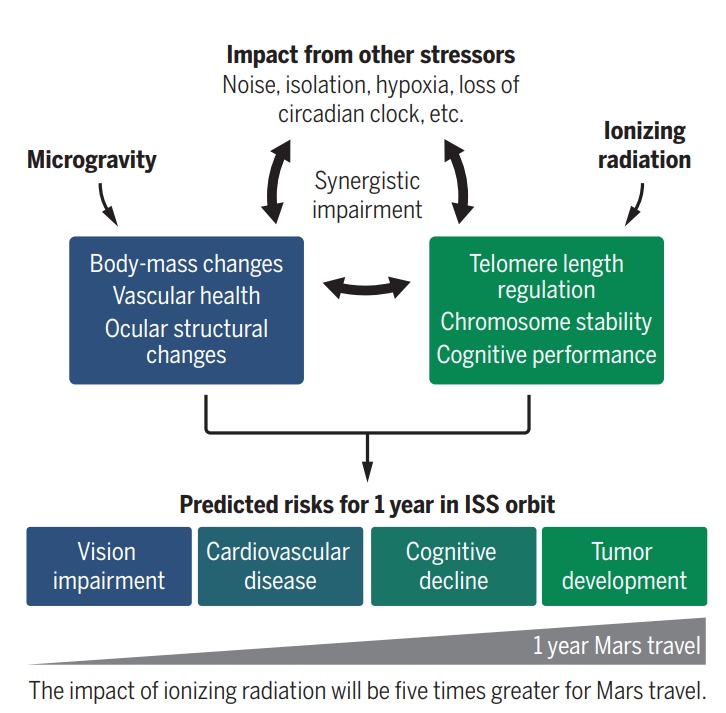
- Environmental challenges in space include:
- Weightlessness (also called microgravity)
- Ionizing radiation exposure
- Noise
- Isolation
- Hypoxia
- Altered nutrition
- Disrupted circadian rhythm

- Known impacts of the spaceflight environment on human health include:
- Reduced red cell mass
- Loss of bone density
- Loss of muscle mass and strength
- Effects on cognitive performance
- Cardiovascular adaptations:
- Headward fluid shift (redistribution of fluids upon entering microgravity)
- Left ventricular mass increase
- Orthostatic tolerance
- Maximal oxygen consumption losses
- Microbial shifts
- Spaceflight-associated neuro-ocular syndrome
- Alterations in gene regulation, including expression of DNA repair genes, reflecting the presence of persistent chromosomal damage
and other stressors. These changes likely impose health risks. Science. 2019 Apr 12;364(6436):127-128
Definition of space anemia:
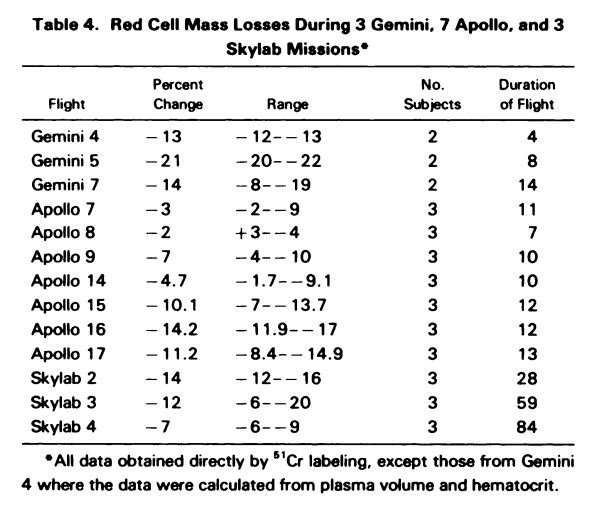
The most consistent hematological finding during the orbital flights has been a significant loss of red cell mass. Typically, there is a 10–12% reduction in red blood cell (RBC) mass occurring in the first 10 days in space (inflight). As we shall see, there is a cyclical change in Hb/Hct as astronauts enter microgravity and then return to earth.
- Early on in flight there is a rapid loss of plasma volume early resulting in hemoconcentration.
- This is followed by a compensatory loss of red cell mass which typically normalizes the Hb.
- Finally upon landing a rapid expansion of plasma volume after landing which unmasks the reduced RBC mass in the form of anemia.
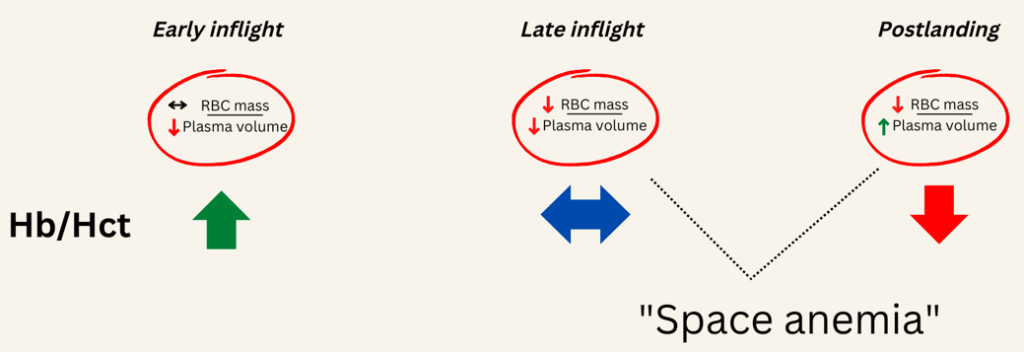
The space community uses the term space anemia in two ways:
- To describe the inflight reduction in RBC mass regardless of the Hb/Hct:1
- Most hematologists would reject this use of the term anemia.
- According to this definition, an astronaut may have relative polycythemia and space anemia at the same time (early in flight, when red cell mass is just beginning to decrease as a compensation for loss of plasma volume)!
- To characterize the the reduction in Hb concentration that develops postflight, after astronauts have landed.
- The term is often used to describe a reduction in Hb even if it does not meet the WHO definition of anemia.
- This caveat notwithstanding, this is a more widely accepted definition of anemia, though
History:
- 1960s, a study on Gemini astronauts demonstrated:2
- Decreased plasma volume during two of these flights.
- Reduced red blood cell (RBC) mass after returning from space.
- Erythrocyte 31Cr survivals determined during Gemini V and VII showed evidence of shortened survival times in three astronauts which is presumptive of a hemolytic state.
- 1980s – 1990s – Lower hemoglobin (Hb) concentrations were reported in 10 astronauts after 9-to-14-day missions. It was estimated that the astronauts lost roughly 1% of their Hb per day in space.3
- By contrast, astronauts on 180-day missions onboard the International Space Station (ISS) were reported to have normal Hb, seemingly contradicting the hypothesis that space flight leads to anemia.4
Epidemiological data:
1. Spacelab Life Sciences missions 1 and 2 (SLS-1 and SLS-2), Spacelab-1 and Space Transport System 41-B:
- Plasma volume decreased by 17% during the first day of flight.
- Red blood cell mass (RBCM) reduction was linearly correlated with mission length; extrapolation to day zero of flight suggested a faster RBCM decrease during the first days of flight.
- Decreased serum erythropoietin (EPO) by about 25%, but only at days 2–4 in-flight, levels returned to normal at flight days 8–12.
- Ferrokinetic data obtained by injecting 59Fe: New RBC production in the bone marrow was not decreased from pre-flight values, and therefore this could not account for the magnitude of the decrease in RBCM. The authors argued that RBCM must have originated from an accelerated RBC destruction with respect to the physiological value (approximately 1%/day).
- 51Cr random labeling studies (percent change in 51Cr specific activity with respect to the value at time of injection was used as an estimate of RBC production and survival):
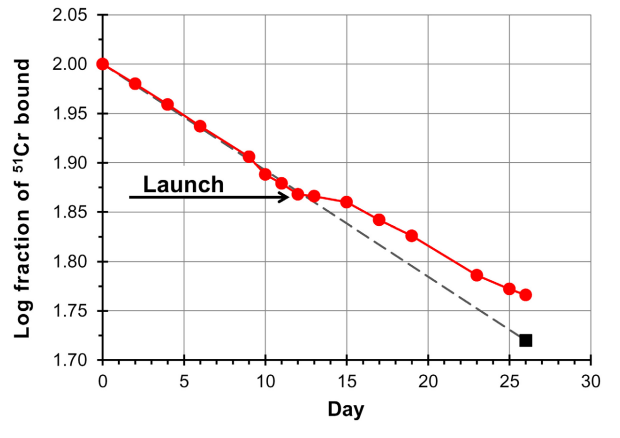
2. A systematic review of space flight hematological parameters (a total of 17,336 hemoglobin (Hb) concentration measures from 721 space missions and controls between 1968 and 2015) revealed the following:
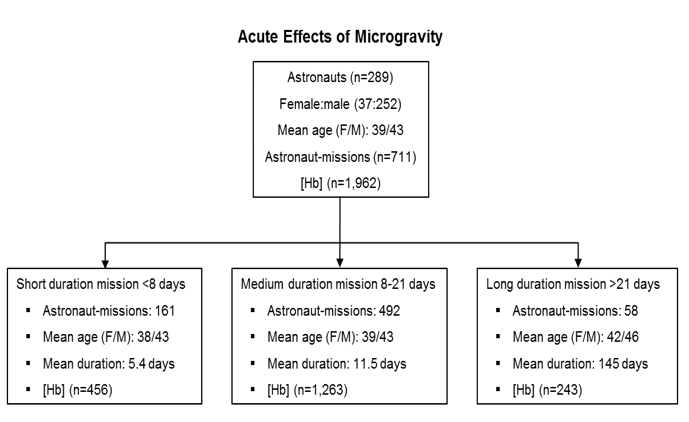
- 12.9% of astronaut-missions reached the WHO definition of anemia on at least one post-flight blood draw, demonstrating dependency of space anemia on the duration of exposure to space:
- 7% of short duration missions.
- 10% of medium duration missions.
- 48% of long duration missions.
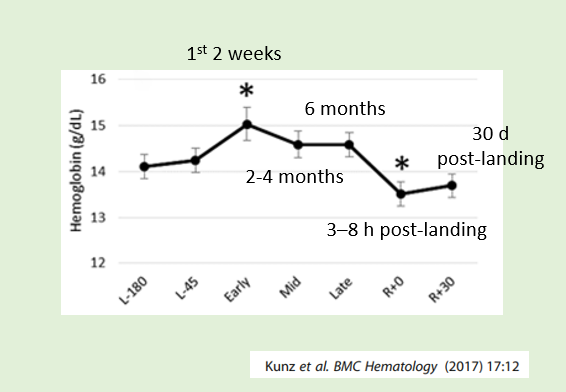
- Astronauts landing after short and medium-duration missions had a higher average Hb compared to preflight, while astronauts landing after long duration missions presented lower average Hb compared to preflight (Mean Hb reduction of 0.74% per day after missions averaging 5.4 days, and of 0.43% per day after missions averaging 11.5 days).
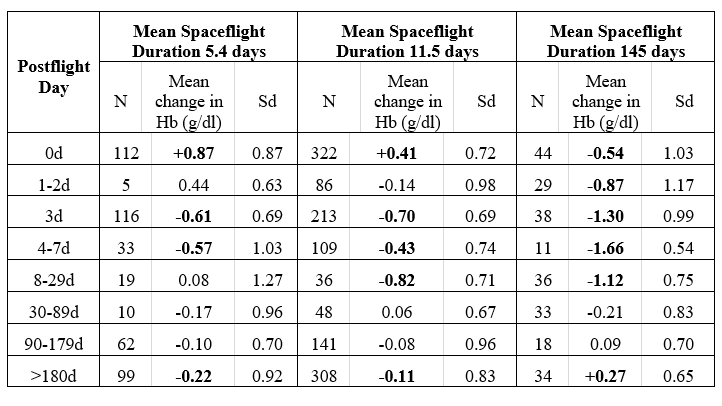
- In the days immediately after landing, all three mission duration groups experienced a progressive reduction in Hb, and by day 3 all were below preflight values:
- Nadir Hb for the short duration group occurred on day 3: a−0.61 g/dL (−0.74,-0.48), or 4.1% reduction compared to preflight
- Nadir Hb for the medium duration group occurred in the 8 to 29 days interval: a−0.82 g/dL(−1.05,-0.58), or 5.4% reduction from preflight
- Nadir Hb for the long duration group occurred in the 4-7 day interval: a−1.66 g/dL (−1.97,-1.34), or 0.9% reduction from preflight.
- The longer the exposure to space, the lower the nadir Hb.
- Recovery from Hb decrements was completed by the 8 to 29-day interval for the short duration mission while the medium and long duration astronaut-missions recovered by the 30 to 89-day interval.
- While Hb returned to preflight levels within about 7 weeks, the longitudinal models predicted that cumulative exposure to space led to small permanent reductions in Hb, proportional to the exposure to space.
Pathophysiology:
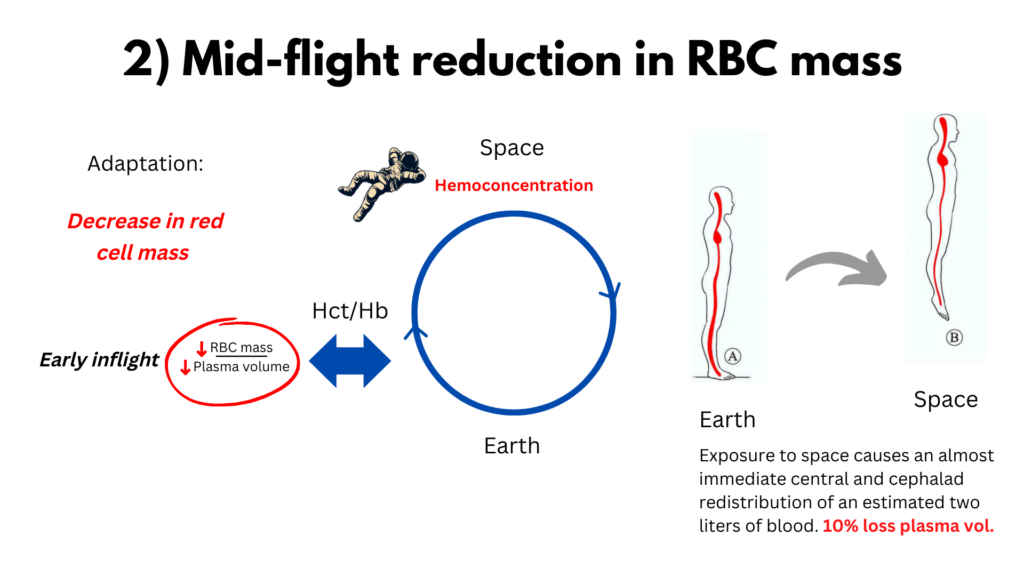
1) Fluid shifts – Exposure to space causes an almost immediate central and cephalad redistribution of an estimated two liters of blood (the peripheral blood normally held in place by gravity, moves to central organs where a condition of acute plethora ensues), which rapidly reduces plasma volume by 10-20%, causing a relative polycythemia.
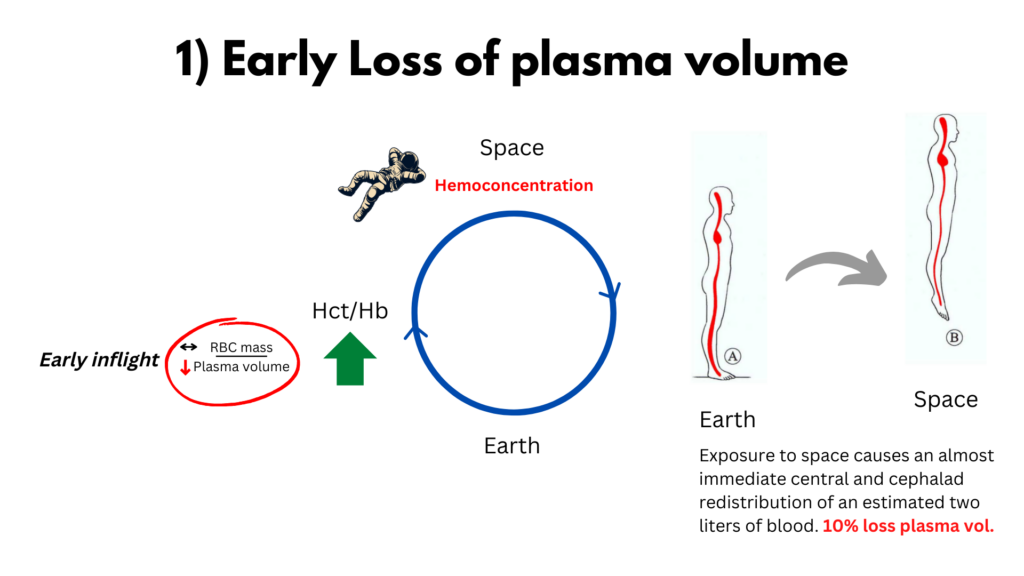
2) The body’s adaptation to the loss of plasma fluid is to reduce red cell mass, which results in normalization or near normalization of the Hb/Hct. Researchers have invoked two potential two mechanisms:
- First is a reduction in the rate of erythropoiesis (in fact, EPO levels decreased significantly, by about 25%, but only at days 2–4 in-flight).
- Second – and perhaps more controversially – is the selective destruction of young red cells in the circulation, a process that has been termed neocytolysis. Because of the long lifespan of RBCs (120 days), it has been argued that a reduction in erythropoiesis cannot account for the observed reduction in RBCM of >10% in ~10 days, and that another mechanism (neocytolysis) must be responsible. A similar mechanisms has been invoked for people returning to sea level from high altitude.
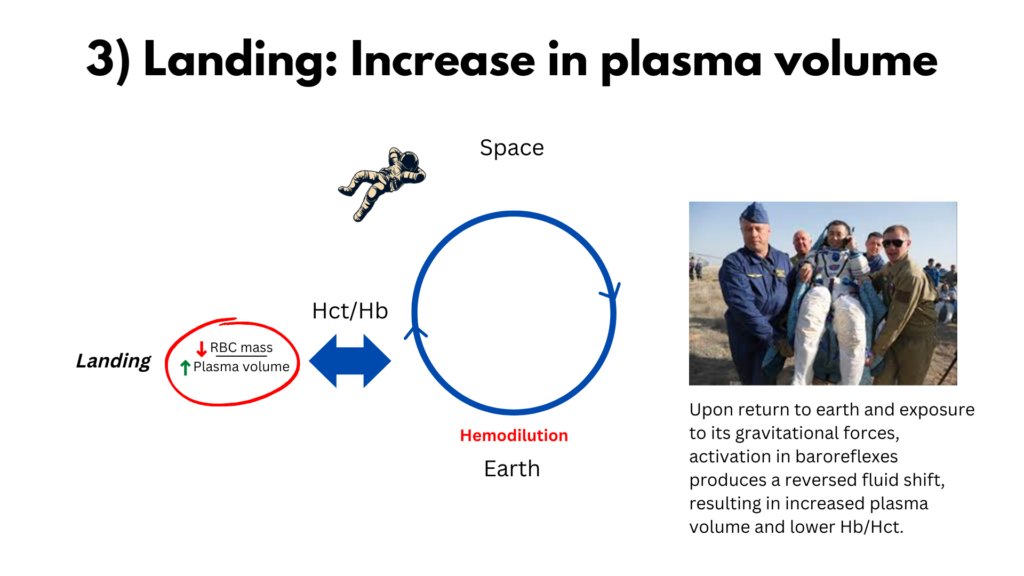
3) Finally, upon return to earth and exposure to its gravitational forces, activation in baroreflexes produces a reversed fluid shift, resulting in increased plasma volume. During this window, hemodilution lowers the Hb level to a nadir, effectively unmasking RBC decrements when compared to preflight Hb. From this nadir on, enhanced EPO-mediated erythropoiesis progressively corrects the RBC decrements.
4) Hemolysis
- Exposure of the American crews to hyperoxia resulting in enhanced red blood cell destruction. A decrease in RBCM of variable amounts was a consistent finding in astronauts during space flights. It was originally imputed to oxidation-induced hemolysis or oxygen inhibition of erythropoiesis, because an atmosphere of pure oxygen was breathed aboard of Gemini or Apollo missions. It was however observed also in subsequent missions where the atmosphere was similar to air at sea level, pointing therefore to a gravity-dependent mechanism capable of altering RBC survival.
- Recent work has pointed to a potentially important for for hemolysis (distinct from neocytolysis) in mediating space anemia (see Nature Medicine paper below).
5) Other proposed mechanisms
- Ineffective erythrocyte production or egress from the bone marrow
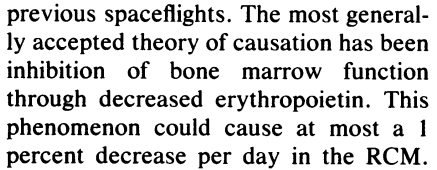
- Low EPO levels or sensitivity
- Abnormally-shaped erythrocytes
- Sequestration in the spleen
- Blood loss
- Nutritional Deficiencies
Trudel et al, Nature Medicine 2022

- A total of 14 astronauts were recruited between 2015 and 2020
- 11 men and 3 women; 45±7 years
- The astronauts flew ISS missions of 167±31 days duration.
- Primary and secondary outcomes were focused on hemolytic parameters:
- End points:
- Primary:
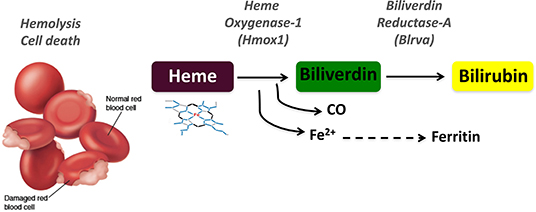
- Carbon monoxide (CO) elimination
- Quantification of CO molecules eliminated used as a direct measure of hemolysis.
- Carbon monoxide (CO) elimination
- Secondary:
- Iron
- Bilirubin
- Transferrin percent saturation
- Ferritin
- Haptoglobin
- Hemolysis markers in breath and blood samples from astronauts preflight, four times inflight and up to 1 year after their 6-month missions to the International Space Station (ISS).
- Primary:
- Results:
- Carbon monoxide (CO) elimination:
- Mean increase from preflight at day 5 was 56%, leading the authors to conclude that “Large elevations in heme degradation products both in alveolar air and blood samples constitute the first direct demonstration of upregulated hemolysis in space and support the hypothesis that space-related anemia is a hemolytic condition.”
- CO elimination in space over 6 months (average of 46 measures) was increased 54% compared to preflight leading the authors to conclude that “Persistently increased CO elimination in space showed that hemolysis was not an acute adaptation to hemodynamic alterations upon entering microgravity. Increased hemolysis rather constituted a primary effect of exposure to space.”
- Sharp decrease in CO elimination 4 days after landing on earth, pointing to a sharp decrease in hemolysis.
- Iron, TSAT and ferritin all showed significant inflight increases compared to baseline, and these quickly normalized upon landing, leading the authors to conclude that “The finding of increased CO elimination was corroborated by the observation that serum iron and iron carrier proteins transferrin and ferritin also increased.”
- The astronaut cohort had an 8.8% decrease in hemoglobin concentration 4 days after landing compared to preflight with 5 of 13 astronauts (38.5%) reaching clinical levels of anemia.
- Carbon monoxide (CO) elimination:
Bottom line:
- Space flight is associated with a:
- Early reduction in plasma volume
- Compensatory reduction in red cell mass (which some in the space community refer to as space anemia, though for most hematologists, this is considered misnomer)
- Depending on the relative change in red blood cell mass and plasma volume, the Hb may:
- Increase (especially on short missions)
- Stay the same
- After landing, astronauts develop postflight reduction in Hb, often reaching levels of anemia. The term space anemia should be reserved for this phenomenon.
- Space flight is also associated with hemolysis:
- In earlier reports, this was called necrocytolysis, and referred to a compensatory mechanism to reduce red blood cell mass as plasma volume contracts during the first few days in space.
- In the more recent Nature Medicine study, hemolysis appears to be a sustained phenomenon even months after landing back on Earth.
The Blood Project reached out to Dr. Guy Trudel about the relationship between the previously described necrocytolysis and the hemolysis he described in his 2022 Nature Medicine study. Here is his response:
“We believe the results we obtained do not support the neocytolysis hypothesis as the likely mechanism for space hemolysis/anemia. Here are 2 reasons:
1) The neocytolysis hypothesis described a rapid self-limited hemolysis initiated by a state of hemoconcentration (in space, caused by fluid shift) to return RBC concentration to normal in space. Therefore, hemolysis would be secondary to fluid shifts. We measured enhanced hemolysis while a mild hemoconcentration remained in space. Hemolysis appears to be a primary effect of being in space/microgravity.
2) The neocytolysis hypothesis, as per its name calls for destruction of young erythrocytes, secondary to low EPO (again inhibited by hemoconcentration). That could potentially work for short-lived hemolysis. We measured ongoing enhanced hemolysis. First, should young erythrocytes be the culprit, their ongoing preferential destruction would rapidly lead to a severe aregenerative anemia. Secondly, suppression of EPO and of EPO-dependent RBC precursors would not increase CO (they have not incorporated Hb yet). Thirdly, hemolysis persisted despite restored-high EPO levels in the second half of the space missions.
However, which subgroup of RBC is preferentially destroyed is space remains to be experimentally demonstrated.”
Guy Trudel, October 4, 2022