Postscript
Introduction
- Iron is an essential trace element.
- Iron is incorporated into numerous proteins, including:
- Hemoglobin
- Catalases
- Lipoxygenases
- Ribonucleotide reductase
- Cytochromes
- Iron is involved in many biochemical processes, including:
- Oxygen transport
- Energy production
- DNA synthesis
- Immune defense
- Gene regulation
- Steroid and hormone synthesis
- Drug metabolism
- Iron deficiency is highly prevalent in patients with chronic kidney disease (CKD), especially those on hemodialysis.
- CKD may be associated with defects in iron homeostasis including:
- Absolute iron deficiency from:
- Increased iron loss
- Reduced iron intake
- Reduced iron absorption
- Functional iron deficiency from:
- Increased iron storage
- Reduced mobilization of iron
- Increased iron needs (with ESA treatment)
- Absolute iron deficiency from:
- The diagnosis of iron deficiency in CKD can be difficult. While diagnostic serum ferritin values of iron deficiency in patients with renal failure are greater than those in patients with normal renal function, there is a large discrepancy in the cut points obtained in various studies published in the literature.
- Iron therapy is an integral part of anemia management in CKD, particularly among patients on hemodialysis. However, considerable uncertainty persists about the initiation, optimal dosing, and monitoring of iron therapy.
Systemic iron homeostasis
- Normal:
- Absorbed dietary iron (~1–2 mg/day)
- Iron loss (~1–2 mg/day)
- Total body iron content is approximately 2–5 g:
- Bone marrow (~300 mg)
- Circulating red blood cells (~1,800 mg)
- Liver parenchyma (~1,000 mg)
- Reticuloendothelial macrophages (~600 mg)
- Transferrin (~3 mg)
- Muscle and other tissues (~400 mg)
- About 25 mg iron recycled from macrophages to erythrocyte precursors each day.
- Chronic kidney disease (for explanation, see Pathophysiology below):
- Absorbed dietary iron (<1 mg/day)
- Iron loss (~5.5–8 mg/day; assumes 2–3 g/year)
- Total body iron content is approximately 2–5 g:
- Bone marrow (~300 mg)
- Circulating red blood cells (~1,400 mg) (assumes reduction in Hb of 3 g/dL)
- Liver parenchyma (~1,000 mg)
- Reticuloendothelial macrophages (~800 mg)
- Transferrin (~3 mg)
- Muscle and other tissues (~400 mg)
- IV iron (~200 mg/month)
- About 25 mg iron recycled from macrophages to erythrocyte precursors each day.
Definitions
- CKD staging based on GFR category
- G1 – GFR > 90 mL/minute/1.73 m2 (normal or high)
- G2 – GFR 60-89 mL/minute/1.73 m2 (mildly decreased compared to young adult level)
- G3a – GFR 45-59 mL/minute/1.73 m2 (mild-to-moderately decreased)
- G3b – GFR 30-44 mL/minute/1.73 m2 (moderate-to-severely decreased)
- G4 – GFR 15-29 mL/minute/1.73 m2 (severely decreased)
- G5 – GFR <15 mL/minute/1.73 m2 (kidney failure)
- Anemia:
- Defined by hemoglobin (Hb) levels according to the Centers for Disease Control and Prevention and World Health Organization:
- For menstruating adults Hb <12 g/dL (120 g/L)
- For pregnant adults Hb <11 g/dL (110 g/L)
- For male adults Hb <13 g/dL (130 g/L)
- These thresholds are accepted by 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.
- Defined by hemoglobin (Hb) levels according to the Centers for Disease Control and Prevention and World Health Organization:
- In patients with CKD, the unavailability of iron for hematopoiesis can be absolute or functional:
- Absolute (or storage) iron deficiency:
- Deficiency of circulating iron that limits erythropoiesis and is caused by deficit of total body iron.
- Functional (or relative) iron deficiency:
- Deficiency of circulating iron that limits erythropoiesis despite normal or elevated body iron stores.
- Absolute (or storage) iron deficiency:
- Given that disorders in both iron balance and iron distribution can impact iron availability, “iron deficiency” (alternatively referred to as “iron restriction”) should be viewed as a mismatch between iron supply and demand, rather than as an indicator of reduced body iron content.
Epidemiology
- In non-dialysis chronic kidney disease (CKD) patients:1
- 21%–62% have anemia, defined as Hb <12 g/dl or <12 g/dl in females and <13.5 g/dl in males, with increasing prevalence in more advanced CKD.
- 15%–72.8% have either ferritin <100 mcg/L (ng/mL) or TSAT <20%, and 8%–20% have both parameters below the threshold.
- In CKD patients on hemodialysis:2
- 64.5%, 14.4%, and 6.6% have Hb levels between 10–12 g/dl, 9 and 10 g/dl, or below 9 g/dl, respectively.
- 15.8% have TSAT <20%, and 4.9% have ferritin <200 mcg/L (ng/mL).
- The Third National Health and Nutrition Examination Survey:
- Prevalence of anemia attributable to CKD in the USA was 390,000 in men and 1.2 million in women.
- Of those with creatinine clearance between 20 and 30 ml/min, 19% of men and 46% of women had a trans-ferritin saturation (TSAT) <20%, while 44% of men and 47% of women in the same kidney function category had a serum ferritin <100 mcg/L (ng/mL).
Pathophysiology
- Effective erythropoiesis in patients with chronic kidney disease (CKD) requires adequate body iron and availability of that iron to the bone marrow.
- Impaired availability of iron for hematopoiesis in patients with CKD can be absolute and/or functional:
- Absolute iron deficiency:
- Advanced CKD is associated with a negative iron balance due to reduced dietary intake, impaired enteral absorption, and increased losses.
- As a consequence of increased losses and decreased absorption, most untreated patients with CKD have reduced iron stores and will progress to a state of absolute iron deficiency unless treated with transfusion or iron supplementation.
- Causes include:
- Decreased intake owing to reduced protein intake secondary to decreased appetite and adherence to low-protein diets.
- Decreased gastrointestinal absorption, caused by:
- Increased levels of hepcidin.
- Concomitant use of certain medications, such as proton pump inhibitors.
- Increased blood loss:
- High rate of iron loss (1–3 g/year) in CKD patients (each milliliter of packed erythrocytes contains 1 mg of iron).
- Patients with CKD not on hemodialysis (HD) and those on HD have been shown to experienced 3.15 and 6.27 mL/day of GI blood losses, respectively, compared to 0.83 mL/day in control subjects.
- Causes include:
- Gastrointestinal bleeding from:
- Gastritis
- Uremia-associated platelet dysfunction
- Iatrogenic loss from:
- Serial blood draws
- Access-site and circuit issues during the dialysis procedure
- Gastrointestinal bleeding from:
- Functional iron deficiency:
- Represents a supply/demand mismatch for iron to support erythropoiesis:
- On the supply side, increased hepcidin leads to iron sequestration in macrophage stores and reduced ability to recruit iron stores from reticuloendothelial cells and hepatocytes for erythropoiesis:
- Hepcidin levels are increased in CKD and negatively correlate with the glomerular filtration rate (GFR).
- The mechanisms responsible for this phenomenon include:
- Increased inflammatory cytokines (the primary mechanism)
- Reduced renal clearance
- On the demand side, erythropoiesis-stimulating agents (ESAs) used in patients with CKD accelerate red blood cell production and exceed the ability to sufficiently mobilize iron from stores (which are otherwise adequate). This leads to a supply/ demand mismatch and a “relative” iron deficiency.
- On the supply side, increased hepcidin leads to iron sequestration in macrophage stores and reduced ability to recruit iron stores from reticuloendothelial cells and hepatocytes for erythropoiesis:
- Represents a supply/demand mismatch for iron to support erythropoiesis:
- Absolute iron deficiency:
Diagnosis
- Overview
- There are two important and distinct aspects of the assessment of iron status testing:
- The presence or absence of storage iron.
- The availability of iron to support ongoing erythropoiesis.
- Tests for iron status:
- Storage iron:
- Serum ferritin:
- Assessment of serum ferritin is the test of choice for iron deficiency.
- However, ferritin is an acute-phase reactant that increases under conditions of inflammation independent of actual iron stores.
- Serum ferritin:
- Availability of iron to support ongoing erythropoiesis:
- Mean cell volume (MCV)
- Transferrin saturation (TSAT)
- % hypochromic red blood cells
- Reticulocyte Hb content (CHr or Ret-Hb)
- Other tests including:
- Soluble transferrin receptor (sTR) assay
- Zinc protoporphyrin assay
- Serum hepcidin assay
- Bone marrow exam (stain for iron):
- The gold-standard test for iron deficiency.
- However, impractical test for routine use.
- Storage iron:
- There are two important and distinct aspects of the assessment of iron status testing:
- Standard tests
- The definitions and diagnosis of iron deficiency are historically based on:
- Serum transferrin saturation (TSAT)
- An indicator of circulating iron.
- TSAT = plasma iron divided by the total iron-binding capacity × 10.
- Serum ferritin, an indicator of stored iron.
- Serum transferrin saturation (TSAT)
- The laboratory criteria used to define iron deficiency and provide indication for treatment are different in patients with chronic kidney disease (CKD) compared to those with normal renal function.
- In those with normal renal function:
- Diagnosis of iron deficiency typically based on:
- Serum ferritin levels <15-100 mcg/L (ng/mL) (depending on the presence of concomitant inflammation).
- Transferrin saturation (TSAT) <16%-20%.
- Diagnosis of iron deficiency may be supported by:
- Decrease in mean corpuscular volume (<80 fL).
- Increase in total iron-binding capacity (>68 mmol/L).
- Increase in percentage of hypochromic red blood cells (HRC) (>6%).
- Decrease in reticulocyte Hb content (CHr) or Ret-Hb (<29 pg).
- Diagnosis of iron deficiency typically based on:
- In those with chronic kidney disease:
- The diagnosis of iron-deficiency anemia in CKD may be challenging.
- While both ferritin concentration and transferrin saturation decline in iron-deficiency anemia, the thresholds of ferritin and transferrin at which iron stores are deficient are higher in patients with CKD compared to those without CKD:
- Ferritin is a positive acute-phase reactant; therefore frequently elevated in patients with CKD, irrespective of their iron stores.
- Transferrin is a negative acute-phase reactant; its concentrations decrease in patients with inflammation.
- Hence, the specificity of low ferritin is high for absolute iron deficiency, but normal or elevated ferritin levels to not exclude iron deficiency in patients with CKD.
- The evidence for these higher thresholds derives from studies comparing serum ferritin and TSAT levels with:
- Absence or presence pf bone marrow iron stores.
- Response to iron therapy
- Definition of absolute iron deficiency:
- In patients with CKD not on hemodialysis therapy, both:
- Transferrin saturation (TSAT) <20%
- Ferritin level <100 mcg/L (ng/mL)
- In patients with CKD on hemodialysis therapy, both:
- Transferrin saturation (TSAT) <20%
- Ferritin level <200 mcg/L (ng/mL)
- In patients with CKD not on hemodialysis therapy, both:
- Definition of functional iron deficiency:
- In patients with CKD not on hemodialysis therapy, both:
- Transferrin saturation (TSAT) <20%
- Ferritin level >100 mcg/L (ng/mL)
- In patients with CKD on hemodialysis therapy, both:
- Transferrin saturation (TSAT) <20%
- Ferritin level >200 mcg/L (ng/mL)
- In patients with CKD not on hemodialysis therapy, both:
- What do the guidelines say?
- 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines:
- Serum ferritin values <30 ng/ml (<30 mg/l) indicate severe iron deficiency and are highly predictive of absent iron stores in bone marrow.
- Serum ferritin values >30 mcg/L (ng/mL), however, do not necessarily indicate the presence
of normal or adequate bone marrow iron stores. - Studies assessing ferritin levels above which all or nearly all patients with CKD have normal bone marrow iron stores have produced varied results but most CKD patients, including those who are on HD, will have normal bone marrow iron stores when their serum ferritin level is >300 ng/ml. Even at serum ferritin levels of 100 mcg/L (ng/mL) most CKD patients have stainable bone marrow iron stores.
- In cases where ferritin >30 mcg/L (ng/mL), the TSAT and serum ferritin level have only limited sensitivity and specificity in patients with CKD for prediction of bone marrow iron stores and erythropoietic response to iron supplementation.
- KDIGO guideline does not provide parameters for diagnosing iron deficiency, though they do provide parameters for treating with iron (see Treatment section below).
- Update to 2012 KDIGO guidelines:
- In CKD, absolute iron deficiency has been defined as TSAT <20% and ferritin <100 mcg/L (ng/mL) in patients not on hemodialysis therapy or <200 mcg/L (ng/mL) in hemodialysis patients (the authors reference 4 studies and review articles).
- Functional iron deficiency is defined by TSAT <20% and ferritin level >100 mcg/L (ng/mL) in CKD without kidney replacement therapy and >200 mcg/L (ng/mL) in patients with CKD treated by dialysis.
- The conference participants agreed that the presently used parameters are not reliable for estimating body iron stores or predicting response to therapy.
- European Renal Best Practice (ERBP)
- In 2004, EBPG recommended lower limits of ferritin and TSAT of, respectively, 100 ng/ml and 20%, with target ranges of respectively 200–500 ng/ml and 30–50%. In 2006, and in light of patient safety, KDOQI defined the lower ferritin limit on the basis of CKD status (100 ng/ml in non-HD-CKD and 200 ng/ml in HD-CKD); if serum ferritin levels are >500 ng/ml, iron administration should be discouraged.
- The ERBP Work Group agreed with the recommendations of the KDOQI guidelines.
- Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease:
- Combination of transferrin saturation (TSAT) and serum ferritin recommended if % HRC and reticulocyte Hb content tests not available or the person has thalassemia or thalassemia trait.
- Absolute iron deficiency (ferritin <100 mcg/L [ng/mL]):
- Low serum ferritin is a useful marker to diagnose absolute iron deficiency.
- Normal or high serum ferritin values (≥100 mcg/L [ng/mL]) do not exclude iron deficiency, as it could be due to other causes as infection or inflammation.
- NICE
- If % HRC and reticulocyte Hb content tests are not available or the person has thalassemia or thalassemia trait, use a combination of transferrin saturation (less than 20%) and serum ferritin measurement (less than 100 micrograms/liter).
- 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines:
- The definitions and diagnosis of iron deficiency are historically based on:
- Newer tests
- The reticulocyte Hb content (CHr) and percentage of hypochromic red blood cells (HRC) estimate the Hb content of red blood cells rather than the amount of storage iron, providing a snapshot of recent iron availability for Hb synthesis which acts as a sensitive indicator of functional iron deficiency and are possibly better than TSAT and ferritin in predicting whether or not there will be a response to iron administration:
- Reticulocyte Hb content:
- Indicates whether iron is incorporated into reticulocytes within 3–4 days after starting iron administration and thus serves as a functional parameter that may be useful in guiding iron and ESA therapy.
- Percentage of hypochromic RBC:
- Reflects iron availability in the preceding 2–3 months, making it a sensitive long-term time-averaged functional parameter.
- Reticulocyte Hb content:
- What do the guidelines say?
- Update to 2012 KDIGO guidelines:
- The development and adoption of new tests to more accurately diagnose both absolute and functional iron deficiency, and to monitor response to therapy, is a high priority research area.
- Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease:
- Tests to determine iron status may include:
- Percentage of hypochromic red blood cells (% HRC), but only if processing of blood sample is
possible within 6 h or, - Reticulocyte Hb count (CHr) or equivalent tests e.g. reticulocyte Hb equivalent.
- Percentage of hypochromic red blood cells (% HRC), but only if processing of blood sample is
- Tests to determine iron status may include:
- NICE
- To diagnose iron deficiency and determine potential responsiveness to iron therapy and long-term iron requirements:
- Use percentage of hypochromic red blood cells (% HRC; more than 6%), but only if processing of blood sample is possible within 6 hours.
- If using percentage of hypochromic red blood cells is not possible, use reticulocyte Hb content (CHr; less than 29 pg) or equivalent tests – for example, reticulocyte Hb equivalent.
- To diagnose iron deficiency and determine potential responsiveness to iron therapy and long-term iron requirements:
- Update to 2012 KDIGO guidelines:
- The reticulocyte Hb content (CHr) and percentage of hypochromic red blood cells (HRC) estimate the Hb content of red blood cells rather than the amount of storage iron, providing a snapshot of recent iron availability for Hb synthesis which acts as a sensitive indicator of functional iron deficiency and are possibly better than TSAT and ferritin in predicting whether or not there will be a response to iron administration:
Treatment
- Iron therapy may be undertaken:
- As primary treatment of anemia in patients.
- As an adjuvant to erythropoiesis-stimulating agents (ESAs).
- The benefits of correction of iron repletion include improvements in:
- Hb
- Cognitive function
- Thermoregulation
- Muscular performance
- Ability to fight infections
- The goal is to balance transfusion avoidance with the risks and side effects of iron therapy.
- IV iron has been shown to be significantly more effective than oral iron for replenishing depleted iron stores in patients on hemodialysis. The data related to relative efficacy of IV vs. oral iron in nondialysis patients is less clear.
- What do the guidelines say?
- 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines:
- In patients with CKD-associated anemia, iron supplementation is intended to:
- Assure adequate iron stores for erythropoiesis.
- Correct iron deficiency.
- In patients receiving ESA treatment:
- Improve the erythropoietic response.
- Prevent iron deficiency from developing.
- Iron supplementation, particularly with intravenous iron, can enhance erythropoiesis and raise Hb levels in CKD patients with anemia even when TSAT and ferritin levels are not indicative of absolute iron deficiency, and even when bone marrow studies reveal adequate iron stores.
- For adult patients with CKD stage 3-5 with anemia not on iron or ESA therapy we suggest a trial of IV iron (or in CKD ND patients alternatively a 1–3 month trial of oral iron therapy) if:
- an increase in Hb concentration without starting ESA treatment is desired and
- TSAT is <30% and ferritin is <500 mcg/L (ng/mL).
- For adult CKD patients on ESA therapy who are not receiving iron supplementation, we suggest a trial of IV iron (or in CKD ND patients alternatively a 1–3 month trial of oral iron therapy) if:
- An increase in Hb concentration or a decrease in ESA dose is desired and,
- TSAT is <30% and ferritin is <500 mcg/L (ng/mL).
- “It is the consensus of the Work Group that additional IV iron should not routinely be administered in patients with serum ferritin levels that are consistently >500 mcg/L (ng/mL).”
- However, for selected patients, “a therapeutic trial of additional iron (i.e., a single course of up to 1,000 mg of iron over a period of several weeks which can be repeated as needed) may be undertaken in patients with serum ferritin > 500 ng/ml after due consideration of potential acute toxicities and long-term risks.”
- The guideline notes:
- Evidence to support a recommendation for specific TSAT and ferritin levels at which iron therapy should be initiated or as ‘targets’ for iron therapy is limited, with very few randomized control trials.
- No studies have addressed the clinical benefit, cost-effectiveness, and risk-benefit comparison of using different TSAT and ferritin levels for the diagnosis of iron deficiency or as a trigger for iron supplementation.
- Most CKD patients with serum ferritin levels >100 mcg/L (ng/mL) (>100 mcg/L [ng/mL]) have normal bone marrow iron stores, yet many such patients will also have an increase in Hb concentration and/or reduction in ESA dose if supplemental iron is provided.
- The Work Group sought to recommend iron targets that balance diagnostic sensitivity and specificity with assumptions regarding safety.
- In patients with CKD-associated anemia, iron supplementation is intended to:
- Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease:
- Therapy targets aim at:
- Minimizing the ESA dose required to maintain target Hb levels in patients on ESA therapy.
- Maximizing the Hb level and minimizing the need to initiate ESA therapy to achieve target-range Hb
levels in patients not on ESA therapy.
- Recommend that patients should be iron replete to achieve and maintain target Hb whether receiving ESAs or not. Iron repletion is usually defined as:
- %HRC <6% / CHr >29 pg/ferritin and TSAT (>100 mcg/L (ng/mL) and >20%).
- For children, aim for a target ferritin level greater than 100 microgram/L for CKD patients on dialysis as well as CKD patients not on ESA therapy.
- The evidence behind the statement that TSAT generally should be maintained at greater than 20% stems from a single RCT comparing higher to lower TSAT targets; patients randomized to a target TSAT of 30% to 50% demonstrated a 40% reduction in ESA dose compared with those assigned to a target of 20% to 30%.
- Low serum ferritin is a useful marker to diagnose absolute iron deficiency. Normal or high serum ferritin values (≥100 microgram/L) do not exclude iron deficiency, as it could be due to other causes as infection or inflammation.
- “We recommend that serum ferritin should not exceed 800 mcg/L (ng/mL) in patients treated with iron, and to achieve this iron management should be reviewed when the ferritin is >500 microgram/L.”
- Therapy targets aim at:
- NICE
- In adults, children and young people treated with iron, serum ferritin levels should not rise above 800 mcg/L (ng/mL). In order to prevent this, review the dose of iron when serum ferritin levels reach 500 mcg/L (ng/mL).
- Offer iron therapy to adults, children and young people with anemia of CKD who are receiving ESAs to achieve:
- Percentage of hypochromic red blood cells less than 6% (unless ferritin is greater than 800 mcg/L [ng/mL]).
- Reticulocyte Hb count or equivalent tests above 29 pg (unless serum ferritin is greater than 800 mcg/L [ng/mL]).
- If these tests are not available or the person has thalassemia or thalassemia trait, iron therapy should maintain transferrin saturation greater than 20% and serum ferritin level greater than 100 micrograms/liter (unless serum ferritin is greater than 800 mcg/L [ng/mL]).
- Most adults will need 500 to 1,000 mg of iron (equivalent doses for children) in a single or divided dose depending on the preparation. Intravenous iron should be administered in a setting with facilities for resuscitation.
- 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines:
Primary data
- Studies comparing serum ferritin +/- other iron indices with bone marrow iron stores in patients without CKD:
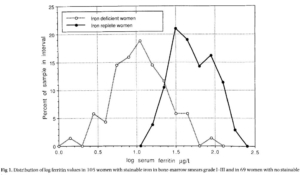
- Hallberg et al:
- Serum ferritin assays and staining for bone marrow iron were performed in 203 adult women.
- 2 groups of patients based on bone marrow findings:
- 69 women with no stainable iron.
- 105 women with clearly visible reticuloendothelial iron.
- Sensitivity and specificity of serum ferritin levels for absolute iron deficiency (no stainable iron in the bone marrow):
- Serum ferritin <16 mg/L:
- Sensitivity 75%
- Specificity 98%
- Serum ferritin <30 mg/L:
- Sensitivity 93%
- Specificity 75%
- Serum ferritin <16 mg/L:
- The lowest serum ferritin value observed among the iron replete women was 14 pg/l, and the 2.5th, 5th. 10th and 50th percentile values were 15, 16, 20 and 42 pg/l, respectively.
- In the women lacking stainable iron, 52/69 (75%) had serum ferritin <15 pg/I.
- In the Irion deficient women the 5Oth, 75th. 90th and 95th percentile values were 9, 15, 26 and 31 pg/l, respectively.
- The best discrimination was obtained at serum ferritin <16 pg/l (specificity 98%: sensitivity 75%).
- Hallberg et al:
-
- Harju et al:
- Serum ferritin assays and staining for bone marrow iron were performed on 123 symptomatic outpatients (male and female) with gastritis, gastric ulcer and duodenal ulcer.
- Bone marrow iron stores were considered “deficient,” “sufficient,” or “plenty.”
- At serum ferritin concentrations <15 and <30 mg/L, sensitivity was 72% and 92%, respectively, and specificity was 96% and 92%, respectively.
- The authors stated: “However, it can be said that 20-25 pg/l forms a rough limit for serum ferritin, below which stainable iron cannot be demonstrated in the bone marrow“.
- Harju et al:
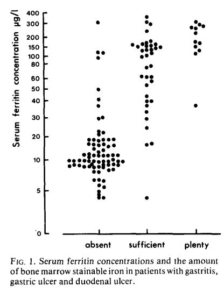
Authors wrote: “However, in the group of ‘absent’ bone marrow iron, some high ferritin values were observed. On the other hand, in the ‘sufficient’ group there were a number of patients who had relatively low serum ferritin values… However, it can be said that 20-25 pg/l forms a rough limit for serum ferritin, below which stainable iron cannot be demonstrated in the bone marrow.”
-
- Jonker et al.
- Serum ferritin (and other iron indices) was compared with staining for bone marrow iron in 87 healthy Malawian children (age 6–66 months).
- Of all children, 44.8% had depleted bone marrow iron stores.
- Using optimized cut-offs, ferritin (<18 mg/L) and sTfR-F (>1.85) best predicted depleted iron stores with a sensitivity/specificity of 73.7%/77.1% and 72.5%/75.0%, respectively.
- Milman et al:
- Serum ferritin was compared with staining for bone marrow iron in 53 healthy students.
- Sensitivity and specificity of serum ferritin levels for absolute iron deficiency (no stainable iron in the bone marrow):
- Serum ferritin <15 mg/L:
- Sensitivity 60%
- Specificity 100%
- Serum ferritin <30 mg/L:
- Sensitivity 100%
- Specificity 89%
- Serum ferritin <15 mg/L:
- Systematic review
- Goal: To determine the diagnostic accuracy of ferritin concentrations (serum or plasma) for detecting iron deficiency and risk of iron overload in primary and secondary iron-loading syndromes.
- Conclusion: “At a threshold of 30 micrograms/L, there is low-certainty evidence that blood ferritin concentration is reasonably sensitive and a very specific test for iron deficiency in people presenting for medical care. There is very low certainty that high concentrations of ferritin provide a sensitive test for iron overload in people where this condition is suspected. There is insufficient evidence to know whether ferritin concentration performs similarly when screening asymptomatic people for iron deficiency or overload.”
- Jonker et al.
- Studies comparing serum ferritin +/- other iron indices with bone marrow iron stores in patients with CKD:
- Stancu et al:
- Serum ferritin and TSAT were compared with staining for bone marrow iron in 100 anemic (hemoglobin <11 g/dL) nondialysis patients with CKD stages 3-5, not receiving erythropoietin stimulating agents (ESAs) or iron.
- Bone marrow iron was judged as depleted versus replete.
- Bone marrow iron stores were depleted in 48% of patients at baseline.
- Inflammation was highly prevalent because 60% of patients had a serum CRP level >10 mg/L.
- Only 17% of patients had TSAT <20% and ferritin level <100 ng/mL.
- One month after 1000 mg of intravenous iron, the mean Hb attained was 10.0 g/dl, with a 1.0 +/- 0.4-g/dl mean increase; Hb increased by 1 g/dl in 48% of patients:
- The proportion of responders was twice as high in patients with iron-deplete versus iron-replete stores (63 versus 30%). NOTE: 30% of patients with adequate iron stores responded.
- Median TSAT and ferritin were lower in responders.
- “Examining various cutoff values, almost none of the selected cutoff values for TSAT, ferritin, and their combination rendered both significant sensitivity and specificity. Only ferritin at a cutoff value of 175 ng/mL yielded significant sensitivity and specificity, both estimated at 71%, which are moderate test characteristics to inform a clinical decision.”
- Stancu et al:
-
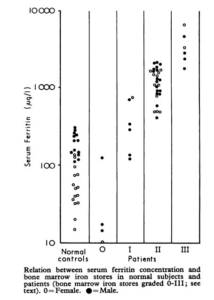
- Kalantar-Zadeh et al:
- Serum ferritin and TSAT were compared with staining for bone marrow iron in 25 anemic (hemoglobin ~11 g/dL) patients with creatinine greater than 3 mg/dL; 20 (80%) were dialysis patients.
- Bone marrow iron was graded 0 to +4, ranging from absent to diffuse homogeneous iron staining.
- The bone marrow:
- Contained essentially no iron in three patients (score 0).
- Had only minimal amounts of stainable iron in seven patients (score +l).
- Had slight to patchy staining in seven other patients (score +2).
- Had patchy to diffuse iron staining in 13 others (scores +3 and +4).
- Scores 0 and + 1 (10 patients) were considered absolute iron deficiency.
- The reference standard indicated that iron deficiency existed in 40% of patients.
- Transferrin saturation of less than 20% was 88% sensitive, but only 63% specific.
- Serum ferritin levels less than 200 µg/L were 87% specific for the diagnosis of iron deficiency anemia, but only 50% sensitive.
- Kalantar-Zadeh et al:


A) Significant correlation (Spearman rank) between serum ferritin concentrations (logarithmic scale) and the semiquantitative bone marrow iron deposit scores in 25 patients. (B) Nonsignificant relationship between serum transferrin saturation and semiquantitative bone marrow iron deposit scores in the same patients.
-
- Fernández-Rodríguez et al:
- Serum ferritin and other iron indices were compared with staining for bone marrow iron in 63 patients undergoing dialysis:
- Twenty-nine patients (19 men, 10 women) were undergoing regular hemodialysis treatment.
- 34 patients (27 men, 7 women) were undergoing CAPD.
- Serum ferritin concentration was the most reliable parameter for the detection of depleted iron stores with the largest area under the ROC curve.
- Mean serum ferritin in those with:
- No iron stores was 156.6 ug/L.
- Iron stores was 421.4 ug/L.
- The positive predictive value of serum ferritin was less than the negative predictive value for almost all cut points studied, including some values less than 100 µg/mL.
- When sensitivity, specificity, and positive and negative predictive values are considered together, serum ferritin values greater than 100 µg/L could be useful to exclude iron deficiency, with a sensitivity and specificity of 75% for a cut point of 121 µg/L.
- Serum ferritin and other iron indices were compared with staining for bone marrow iron in 63 patients undergoing dialysis:
- Fernández-Rodríguez et al:
-
-
- Bell et al:
- Fifty-five patients on maintenance hemodialysis underwent bone marrow aspirations for evaluation
of iron stores that were to be compared to concomitant measurements of hematocrit, red blood cell volume, serum iron concentration, total iron binding capacity, transferrin saturation, and serum ferritin concentration. - In 42 patients (76.4%), the bone marrow iron stores were found to be absent or deficient.
- Of the 55 patients, 42 (76.4%) had either absent or decreased iron stores, whereas 4 (7.3%) had
normal and 9 (16.4%) had increased iron stores. - Average serum ferritin was 178.4 ng/ml (range, 4 to 1800 ng/ml):
- Of those with absent, mean ferritin 27.7 (4-108).
- of those with decreased iron stores , mean ferritin 43.4 (10-127).
- Average transferrin saturation (TSAT) was 22.6 (7-62):
- Of those with absent, mean TSAT 17% (7-43).
- of those with decreased iron stores, mean TSAT 21.3% (11-57).
- Serum ferritin was the best predictor of iron storage levels, with diagnostic thresholds of 80
to 350 ng/ml.
- Fifty-five patients on maintenance hemodialysis underwent bone marrow aspirations for evaluation
- Bell et al:
-
-
-
- Blumberg et al:
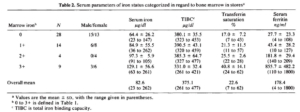
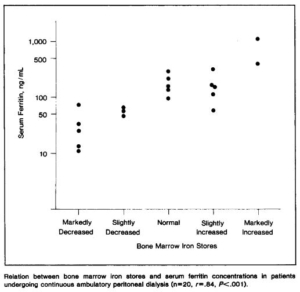
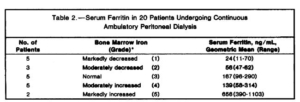
- 20 patients undergoing continuous ambulatory peritoneal dialysis (CAPD).
- serum ferritin concentration (radioimmunoassay) was compared with bone marrow iron stores (graded semiquantitatively).
- Patients with decreased iron stores (8/20) had serum ferritin concentrations below 70 ng/mL; those with normal or increased iron stores had concentrations above 96 ng/mL.
- concluded that serum ferritin concentrations adequately reflect bone marrow iron stores and are useful as a guide to iron-replacement therapy in patients undergoing CAPD. Our results suggest that serum ferritin concentrations also correlate well with stainable bone marrow iron in patients undergoing CAPD.
- Blumberg et al:
-
-
-
- Hussein et al:
- Forty-four patients with chronic renal failure on hemodialysis.
- All received intravenous iron dextran 100 mg on alternate weeks.
- The hemoglobin concentrations of the 44 patients ranged from 4.3 to 16.0 g/dl.
- All the patients were anemic except one male patient with polycystic disease.
- Bone marrow stainable iron:
- None in 4 patients
- Normal in 6
- Increased in 28
- Greatly increased in 6
- In the 44 patients there was a good correlation between the serum ferritin concentrations and the bone marrow stores.
- Serum ferritin was normal in one patient with grade 0 bone marrow iron stores.
- Hussein et al:
-
-
-
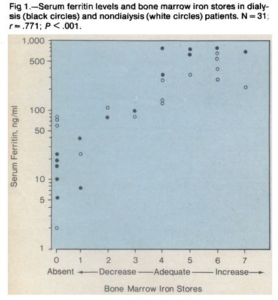
- Mirahmadi et al:
- Seventeen patients without renal failure who had bone marrow examinations as a part of the clinical evaluation and 14 anemic dialysis patients were studied.
- A significant correlation was found between serum ferritin levels and bone marrow iron stores in the dialysis (P <.001) and nondialysis patients (P <.001).
- Nine patients with absent bone marrow iron stores had mean serum and bone marrow ferritin values of 32 ng/ml and 2.3 jug/ml, respectively The patients with adequate or increased bone marrow iron stores had serum ferritin values greater than 120 ng/ml,
- Serum ferritin determinations appear to give an accurate estimation of bone marrow iron stores, thereby providing a reliable guide for iron replacement therapy and reducing the need for repeated bone marrow aspirations.
- Serum ferritin levels of less than 105 ng/ml suggest decreased iron stores, and values greater than 120 ng/ml indicate adequate or increased iron stores.
- On the basis of our findings, patients with serum ferritin levels less than 120 ng/ml probably should receive iron supplementation to replete body iron stores. Conversely, patients with serum ferritin values greater than 120 ng/ml should not receive iron therapy, since bone marrow iron stores are adequate or increased, even though the iron present may not be available for effective erythropoiesis.
- Mirahmadi et al:
-

- Studies examining treatment effect of iron depending on baseline and/or maintenance serum ferritin levels:
-
- FIND-CKD study
- 56-week, open-label, multicenter, prospective and randomized study of 626 patients with nondialysis-dependent CKD, anaemia and iron deficiency not receiving erythropoiesis-stimulating agents (ESAs).
- Inclusion criteria included any ferritin level <100 or <200 μg/L with transferrin saturation (TSAT) <20%, within 4 weeks of randomization.
- Patients were randomized (1:1:2) to intravenous (IV) ferric carboxymaltose (FCM), targeting a higher (400–600 μg/L) or lower (100–200 μg/L) ferritin or oral iron therapy.
- The primary end point was time to initiation of other anaemia management (ESA, other iron therapy or blood transfusion) or hemoglobin (Hb) trigger of two consecutive values <10 g/dL during weeks 8–52.
- The primary end point occurred in 36 patients (23.5%), 49 patients (32.2%) and 98 patients (31.8%) in the high-ferritin FCM, low-ferritin FCM and oral iron groups, respectively [hazard ratio (HR): 0.65; 95% confidence interval (CI): 0.44– 0.95; P = 0.026 for high-ferritin FCM versus oral iron].
- The increase in Hb was greater with high-ferritin FCM versus oral iron (P = 0.014) and a greater proportion of patients achieved an Hb increase ≥1 g/dL with high-ferritin FCM versus oral iron (HR: 2.04; 95% CI: 1.52–2.72; P <0.001).
- PIVOTAL study
- 50-center open-label trial of 2,141 adult patients undergoing maintenance hemodialysis for fewer than 12 months conducted in the United Kingdom between 2013 and 2018.
- Patients randomized to receive either high-dose iron sucrose, administered intravenously in a proactive fashion (400 mg monthly, unless the ferritin concentration was >700 μg per liter or the transferrin saturation was ≥40%), or low-dose iron sucrose, administered intravenously in a reactive fashion (0 to 400 mg monthly, triggered only for TSAT <20% and ferritin <200 mg/l).
- The primary endpoint was the composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or death, assessed in a time-to-first event analysis.
- Secondary endpoints included death, infection rate, and ESA dose.
- Participants in the high-dose group received a median monthly iron dose of 264 mg, compared with 145 mg in the low-dose group.
- Over a median follow-up of 2.1 years, the serum ferritin concentration and transferrin saturation increased from baseline to approximately 650 ng/mL and 27%, respectively, with the high-dose regimen, whereas they remained near baseline levels (200 ng/mL and 22%), with the low-dose regimen
- High-dose intravenous iron regimen administered proactively was superior to a low-dose regimen administered reactively and resulted in lower doses of erythropoiesis-stimulating agent being administered.
- A total of 320 patients (29.3%) in the high-dose group had a primary endpoint event, as compared with 338 (32.3%) in the low-dose group (hazard ratio, 0.85; P <0.001 for noninferiority; P <0.04 for superiority).
- Commentary:
- Li and Kshirsagar
- PIVOTAL is the largest randomized study to prospectively examine the impact of different intensities of IV iron dosing regimens on clinical outcomes for hemodialysis patients
- The treatment protocols in both regimens used relatively conservative thresholds for terminating IV iron dosing. For example, IV iron administration was discontinued in the proactive regimen group when ferritin levels reached >700 μg/L or TSAT reached 40%. Although it is common practice to discontinue IV iron treatment when these levels are >1,200 μg/L and 50%, respectively, definitive recommendations are lacking and vary for IV iron administration for ferritin levels between 800 and 1,200 μg/L and TSATs between 40% and 50%.
- Li and Kshirsagar
- FIND-CKD study
