Postscript
Prev
1 / 1 Next
Prev
1 / 1 Next
Introduction
- Benign ethnic neutropenia (BEN) is an inherited cause of an absolute neutrophil count (ANC) < 1 x 109/L with no clinical sequelae or increased risk of infection that is most often seen in people of sub-Saharan African descent.
- It results from a polymorphism in a gene (atypical chemokine receptor 1, ACKR1) that encodes for Duffy antigen receptor for chemokines (DARC, also called Duffy antigen), a membrane glycoprotein that acts as a chemokine receptor for proinflammatory cytokines. This “Duffy-negative” phenotype is caused by one nucleotide substitution within the promoter of ACKR1, which disrupts the sequence binding the erythroid transcription factor GATA-1 and leads to the selective loss of ACKR1 expression in red blood cells but not endothelium.
What’s in a name?
- Name of the condition:
- Historically, lower absolute neutrophil counts (ANC) were linked with African ancestry, but we now know this to be strongly associated with a single nucleotide polymorphism (SNP) in the ACKR1 gene, with the null phenotype providing a selective advantage against Plasmodium vivax.
- The term benign ethnic neutropenia was originally coined to describe this condition of low ANC in people of African ancestry, but there has been a recent call to change the name. In this regard, both of the following passages are worth quoting in their entirety:
- Merz et al wrote:
- “[First], we propose erasing “benign ethnic neutropenia” from the lexicon and replacing it with the terminology Duffy-null associated neutrophil count (DANC). This change de-pathologizes a common and healthy variant as well as correlates the variant with a biologic marker rather than ethnicity. Second, these data support the dire need for implementation of Duffy null specific ANC reference ranges. Future studies should establish ANC reference ranges for Duffy null individuals and investigate the impact of this variant on other aspects of clinical care such as administration of chemotherapeutics as this would likely improve healthcare for approximately 27 million Americans and ameliorate a significant inequity in medicine.”
- Merz and Achebe wrote:
- “It is no surprise that systemic racism, structures of discriminatory policies and practices that assign privilege on the basis of skin color, is insidiously embedded in systems that we blithely invoke in our daily practice. We need to examine why we ascribe labels to clinically insignificant variants, labeling as conditions differences that predominantly affect non-White communities. Systemic racism was purposely construed; therefore, deliberate actions are crucial to its abolishment. Thus, we advocate for “benign ethnic neutropenia” to be referred to as “typical neutrophil count with Fy(a-b-) status.” This is a more accurate description of the genetic driver of the lower ANCs and avoids the suggestion that ethnicity alone is causal or that this common variant is abnormal. In addition, we anticipate that further research will allow for the development of new comprehensive ANC references ranges that account for the Fy(a-b-) phenotype, which should eliminate the need for a qualifying label altogether. We must actively confront and correct the racist labeling of common and harmless characteristics in non-White people as abnormal conditions simply because they differ from White phenotypes. Rejecting the term “benign ethnic neutropenia” is a simple way to start.”
- The Duffy null phenotype (also referred to as Fy(a-b-) phenotype, which we described below) has stronger diagnostic utility for DANC than self-identified race or ethnicity: Fy(a-b-) phenotype, 97.36% sensitivity, 95.65% specificity Self-identified race, 65.7% sensitivity, 48.8% specificity.
- Merz et al wrote:
- Based on these consideration, The Blood Project has adopted the new name Duffy-null associated neutrophil count (DANC).
- Name of the gene involved:
- Duffy blood group or antigen described 60 years ago.
- Duffy antigen is also known as the Duffy antigen receptor for chemokines (DARC).
- DARC, in turn, is also known as atypical chemokine receptor 1 (ACKR1).
- ACKR1 allele also referred to as FY allele.
Definitions
- Neutropenia is defined as an absolute neutrophil count (ANC) < 1.5 x 109/L.
- Classification of neutropenia:
- Based on severity:
- Mild neutropenia 1.0-1.5 x 109/L
- Moderate neutropenia 0.5-1.0 x 109/L
- Severe neutropenia < 0.5 x 109/L
- Congenital vs. acquired:
- Congenital:
- Associated with increased risk of infection:
- Severe congenital neutropenia
- Cyclic neutropenia
- Not associated with increased risk of infection:
- DANC
- Associated with increased risk of infection:
- Acquired:
- Infections
- Medications
- Autoimmune disorders
- Malignant disease
- Nutritional deficiencies
- Congenital:
- Based on severity:
- DANC is a diagnosis of exclusion, most often identified in individuals of certain ethnicities including African, Middle Eastern and Caribbean in the setting of at least three blood samples showing neutropenia at intervals of at least two weeks and with no other identifiable cause.
- Chemokine effects are mediated by classical G-protein coupled receptors (GPCRs). Atypical chemokine receptors are structurally similar to GPCRs but do not couple to G-proteins and therefore fail to induce the full spectrum of downstream intracellular signals that characterize GPCRs. However ACKRs may transport, present or scavenge chemokines and thus, by different means, effectively regulate chemokine availability in tissue microenvironments.
Epidemiology
- It is well-established that many people with African ancestry have absolute neutrophil counts (ANC) lower than Caucasian individuals.
- Data from the National Health and Nutrition Examination Survey show that African-American individuals have a white blood cell count that is on average 700 cells/mL lower than that in White American subjects. Mean leukocyte counts :
- African-Americans 3.5 k/uL
- European-Americans 4·3 k/uL
- Mexican Americans 4·4 k/uL
- Using neutrophil count cutoff of <1·5 k/uL, African-Americans have a prevalence of neutropenia of 4·5%, compared to 0·74% in European-Americans and 0·48% in Mexican-Americans.
- DANC occurs primarily in the following ethnic groups:
- African
- Caribbean
- Middle Eastern
- West Indian
- Reported prevalence of DANC associated with neutropenia:
- 25–50% in people of African ancestry
- 4.5% in African-Americans
- 10.7% of Arabs
- 11.8% in Yemenite Jews
- 15.4% in Black Ethiopian Jews
- < 1% in the white population living in the US
History
- The Duffy blood group was discovered in 1950 when a previously undefined anti-red blood cell antibody, designated anti-Fya, was discovered in the serum of a multiply transfused patient with hemophilia.
- Anti-Fyb was discovered one year later in a patient 2 days after the birth of her third child.
- Duffy was the first blood group locus to be assigned to an autosomal chromosome.
- DANC was first documented in 1941, when it was observed that neutrophil counts in healthy black sharecroppers in Mississippi were lower than those of white workers living under the same conditions.
- The observation that the mean neutrophil counts of black Americans are lower than those of white Americans was subsequently confirmed.
- While searching for CXCL8-binding protein in blood, researchers at Genentech found that:
- CXCL8 was specifically absorbed by erythrocytes.
- Scatchard binding experiments revealed that the binding was saturable and defined by a high affinity receptor.
- It was not always possible to successfully detect CXCL8 binding to blood samples and it appeared that the blood from these CXCL8 non-responders was always from African American donors.
- The receptor was found to be identical to the human red blood cell antigen called the Duffy antigen.
Pathogenesis
- Cause:
- In African Americans and Yemenite Jews:
- Single nucleotide polymorphism (SNP) (rs2814778) of the atypical chemokine receptor 1 (Duffy blood group) (ACKR1) (previously termed DARC) gene on chromosome 1q22-23.
- Mutation occurs at position −30 (GATA box) of the ACKR1 promoter region (usually in the FYB allele) in which thymine (T) is replaced by cytosine (C).
- Other genetic variants implicated in DANC include polymorphisms in:
- CXCL2 on chromosome 4
- CDK6 on chromosome 7
- CSF3 on chromosome 17
- In African Americans and Yemenite Jews:
- About ACKR1
- There are 2 ACKR1 alleles:
- FYA, which encodes the Fya antigen.
- FYB, which encodes the Fyb antigen.
- The 2 alleles are codominant such that if the FYA is inherited from one parent and the FYB allele if inherited from the other, both gene products, Duffy Fya and Fyb antigens, will be expressed on the red blood cells.
- FYA and FYB alleles:
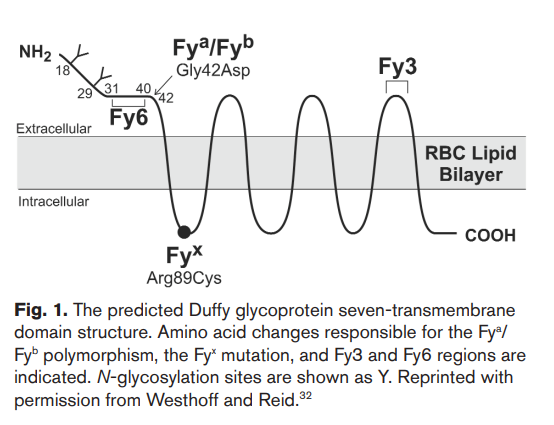
- Differ by a single base substitution in the second position of codon 42 (G125A), resulting in two different encoded proteins, differentiated by a single amino acid, a glycine residue in Fya and an aspartic acid residue in Fyb (Gly42Asp).
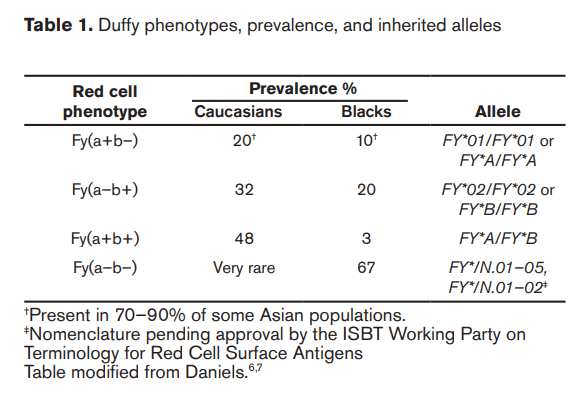
- Combinations of the two alleles can result in three possible phenotypes:
- FYA/ FYB, encoding Fy(a+b)
- FYA/FYA, encoding Fy(ab+)
- FYB/FYB, encoding Fy(a+b+)
- In DANC, a mutation in the promoter region of FYB allele (rarely the FYA allele) results in ACKR1 null phenotype, often represented as Fy(a-b-).
- Individuals harboring this phenotype are designated:
- Duffy negative individuals
- ACKR1-null phenotype
- ACKR1-null allele (FY)
- Fy null-allele
- Null erythrocyte silent’ (ES) phenotype
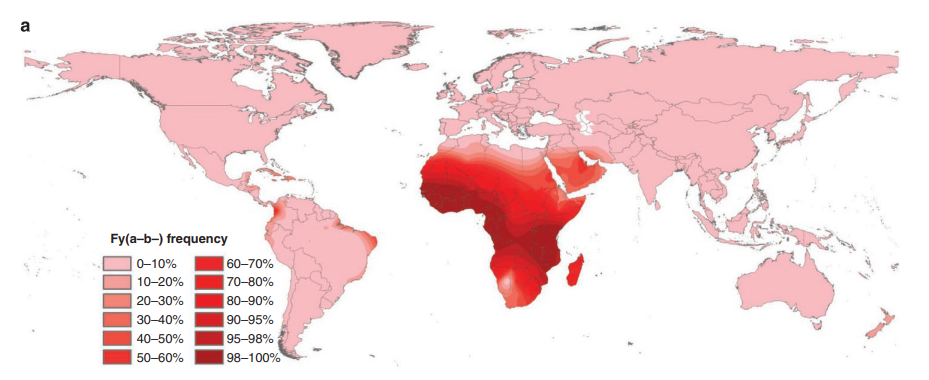
- This phenotype is rare in Caucasians but represents the major allele in Blacks.
- Most common allele worldwide is FYA, while in sub-Saharan Africa, the prevalent allele phenotype is the silent Fy(ab) variant.
- Individuals harboring this phenotype are designated:
- Prevalence of ACKR1 alleles:
- FYA present in:
- 66% of Caucasians
- 10% of Blacks
- 99% of Asians
- FYB present in:
- 83% of Caucasians
- 23% Blacks
- 18.5% of Asians
- 50%-70% individuals from the Arabic peninsula
- Fy(a-b-) phenotype is found in:
- <1% of those with European or Asian ancestry
- 80%-100% individuals from sub-Saharan Africa
- Only 25–50% have ‘neutropenia’
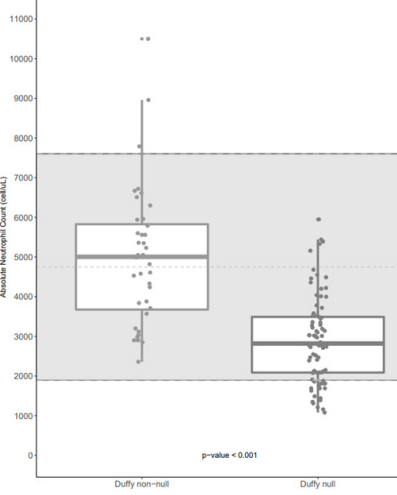
- In a cohort of 120 healthy Black and African American adults, 80 (66.7%) were Duffy negative:
- About 25% Duffy null individuals with ANC below the lower limit of normal (LLN) of 2,000 cells/uL.
- About 10% had ANC<1,500 cells/uL.
- Duffy null individuals had a median ANC over 2,000 cells/uL below the median ANC of Duffy non-null individuals.
- FYA present in:
- There are 2 ACKR1 alleles:
- More on ACKR1/Duffy:
- The ACKR1/Duffy antigen:
- Is a glycosylated seven transmembrane domain receptor protein that functions via a non-G-protein-coupled mechanism
- A promiscuous chemokine binding protein – binds over 20 different CC and CXC chemokines.
- Acts as a non-signaling receptor for pro-inflammatory cytokines and chemokines, resulting in their sequestration and reduced concentration in the circulation.
- ACKR1/DARC/Duffy antigen expression occurs in:
- Red blood cells (RBCs):
- DARC on RBCs is expressed a transmembrane glycoprotein as Duffy blood group antigen, Fy.
- Weakly immunogenic blood group.
- Two phenotypes according to whether the DARC gene is expressed in red blood cells:
- Duffy positive phenotype (expression)
- Duffy null phenotype (no expression)
- Results from homozygous point mutation in the DARC gene
- Mutation in SNP (rs2814778) explains two thirds of the Duffy null phenotype in:
- African Americans
- West Africans
- Yemenite Jews
- The Duffy null phenotype has been reported in less than 3% of whites and in those instances is not attributed to the same SNP polymorphism
- DANC is associated with loss of DARC expression on red cells only; DARC expression in other tissues is preserved.
- Non-erythrocytes:
- Endothelial cells of capillaries and post-capillary venule – ACKR1 in endothelial cells transports chemokines to abluminal (tissue) side.
- Brain – Purkinje cells of the cerebellum
- Kidney – epithelial cells of renal collecting ducts
- Not expressed in white blood cells.
- Red blood cells (RBCs):
- The ACKR1/Duffy antigen:
- Mechanisms include:
- Defect in the release of mature granulocytes from the bone marrow. Supported by:
- Bone marrows showing normal bone marrow cellularity and normal myeloid maturation.
- Observation that hydrocortisone increases neutrophil count, though the bone marrow response is less compared with control subjects.
- Increase in the migration and egress of neutrophils into the organs and tissues. Supported by complete vs. RBC-specific knock-out studies in mice:
- Healthy Duffy-negative individuals of African ancestry have low blood neutrophil counts In contrast, ACKR1-deficient mice had normal neutrophil counts in blood.
- Blood neutrophils in reciprocal wild-type and ACKR1-deficient BM chimeric mice.
- Wild-type mice reconstituted with ACKR1-deficient BM had characteristically altered neutrophils (Supplementary Fig. 8h) and were indeed neutropenic.
- Suggests that DANC not a true neutropenic state and would explain why these individuals are not at increased risk for infection.
- Decrease in the bone marrow granulocyte reserve (little evidence for this mechanism).
- Defect in the release of mature granulocytes from the bone marrow. Supported by:
- Neutrophils in DANC:
- Normal morphology
- Normal gene expression related to antimicrobial function
- Duffy null individuals are hypothesized to have appropriate neutrophil function and response despite lower baseline circulating neutrophils.
- Evolutionary considerations:
- The high frequency of the Duffy null phenotype in African countries where malaria is endemic most likely derives from natural selection for this mutation, as it is protective against Plasmodium vivax, which utilizes the Duffy antigen receptor to enter the red blood cell.
- Duffy antigens are used by malaria merozoites to penetrate and invade red blood cells. In the absence of Duffy antigens [the Fy(a-b-) phenotype], Plasmodium vivax merozoites attach to red blood cells but are unable to invade the cells.
- Thus, the Fy(a-b-) phenotype is protective against malaria and enriched in individuals of sub-Saharan African and Arabic ancestry as this was an advantageous trait.
Clinical presentation
- DANC is largely a diagnosis of exclusion.
- Suspect DANC when individuals of certain ethnicities (especially African, Middle Eastern and Caribbean) present with:
- A persistent absolute neutrophil count < 1.5 x 109/L
- Absence of:
- Symptoms
- Other causes of neutropenia
- It is well established that individuals with DANC are at no increased risk of infection when compared to the general population.
- Presence of recurrent infections, anemia, thrombocytopenia, splenomegaly or lymphadenopathy should raise the concern for other causes of neutropenia.
- There is no threshold value for ANC in a patient suspected to have DANC that should trigger further investigations:
- Current literature does not favor outpatient investigations of neutropenia for individuals of certain ethnicities who have an ANC between 1000 /μL and 1500 /μL, provided they do not suffer from recurrent infections, fever, recurrent or severe oral ulcers, lymphadenopathy, splenomegaly or other cytopenias
- Individuals who have an ANC between 500 /μL and 1000 /μL are recommended to undergo further outpatient investigations to rule out secondary causes of neutropenia.
- It is not necessary to monitor the ANC after diagnosis of DANC.
- In cohort of 46 patients with DANC:
- Their leukopenia resulted from isolated neutropenia, ranging from 1000 and 1500 cells/uL.
- The severity of infections was mild and the frequency was similar to other healthy individuals in the ambulatory clinic.
- In cohort of 120 healthy self-identified Black individuals:
- 66.7% (80/120) had the Duffy null phenotype.
- 23.8% (19/80) of Duffy null individuals had an ANC<2,000 cells/uL compared to no Duffy non-null individuals.
Prev
1 / 1 Next