Postscript
Introduction
Chronic excessive alcohol use has several effects on the hematological system. These include:
- Changes in red blood cells
- Bone marrow:
- Vacuolization of erythroid lineage
- Ring sideroblasts
- Peripheral blood:
- Mild anemia
- Macrocytosis
- Stomatocytosis
- Changes in white blood cells:
- Bone marrow:
- Vacuolization of granulocyte lineage
- Peripheral blood:
- Neutropenia
- Lymphopenia
- Functional alterations in granulocytes
- Bone marrow:
- Changes in platelets:
- Thrombocytopenia
- Bone marrow:
Several themes emerge from the literature:
- Hematological alterations may vary depending on:
- Quantity of alcohol consumption.
- Duration of alcohol consumption.
- Pattern of alcohol consumption:
- Acute and chronic ethanol intoxication may have synergistic, additive or opposing effects.
- Although cytopenias are common, they are:
- Not progressive
- Rarely fatal
- Reversible with abstinence
- There are 2 subtypes of chronic alcoholics, and each may be associated with a distinct hematological phenotype:
- The so-called skidrow alcoholic, who:
- Is often admitted to hospital with alcohol intoxication.
- Has a poor diet and is at increased risk for folate deficiency
- Demonstrates all possible hematological changes.
- Has mortality rate between 8% and 14%.
- Patients who consume excess alcohol while still eating normally and functioning within their families and communities:
- Effects of alcohol may be less dramatic.
- Usually no accompanying folate deficiency.
- The so-called skidrow alcoholic, who:
- The mechanisms by which ethanol exerts its effects on blood cells may include:
- Direct toxic effects of alcohol on:
- Circulating blood cells
- Bone marrow precursor cells
- Indirect effects:
- Poor nutrition
- Infection
- Liver disease
- Direct toxic effects of alcohol on:
| Prevalence | Resolution post abstinence | Comments | |
|---|---|---|---|
| Red blood cells | |||
| Vacuolization | 25% | 3 to 7 days | No obvious functional sequalae |
| Ring sideroblasts | 5-30% | 5 to 10 days | May lead to anemia, though no strong evidence |
| Anemia | 50% | ND | Many different causes |
| Macrocytosis | 70% | 2-4 months | MCV typically < 110 fL |
| Stomatocytosis | 40% | ND | May lead to shortened red cell lifespan |
| White blood cells | |||
| Vacuolization | Less common vs. erythroid precursors | ND | |
| Leukopenia | 5% | ND | Especially in those with bacterial infection |
| Lymphopenia | ND | ND | Mostly T cell depletion |
| Platelets | |||
| Thrombocytopenia | 40% | One week | Rarely at risk for bleeding |
| Thrombocytosis | 15% | ND | May occur as rebound following thrombocytopenia |
Population studies
- A study of 144 consecutive general hospital patients undergoing bone marrow examinations due to abnormalities in peripheral blood counts:
-
- Two populations:
- 57 patients classified as alcohol abusers:
- History of ethanol consumption > mean 60 g/day.
- Before sampling, these patients had been actively drinking for 1-2 weeks in amounts> mean of 120 g/day.
- No evidence of severe liver dysfunction.
- 87 nonalcoholic patients, including:
- Nondrinkers
- Social drinkers with a mean ethanol consumption of less than 20 g/day or 40 g on any single occasion.
- 57 patients classified as alcohol abusers:
- Two populations:
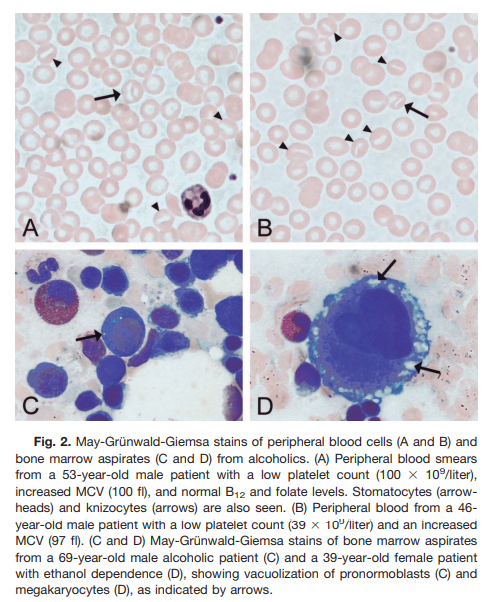
(instead of oval) macrocytes, stomatocytes (41%), and knizocytes (33%) (Fig. 2A and 2B). The incidences of stomatocytosis and knizocytosis in the nonalcoholic group were only 12 and 4%, respectively. Excessive vacuolization of megakaryocytes in the cell periphery was observed in 20% of the alcoholic patients, all of whom were patients with recent intoxication (Fig. 2C and 2D), whereas in the nonalcoholics such morphological features were found in only one patient.
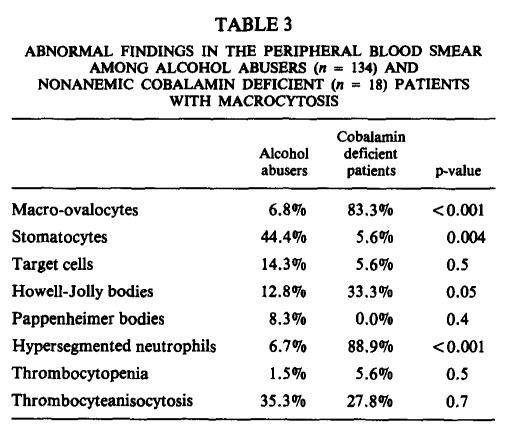
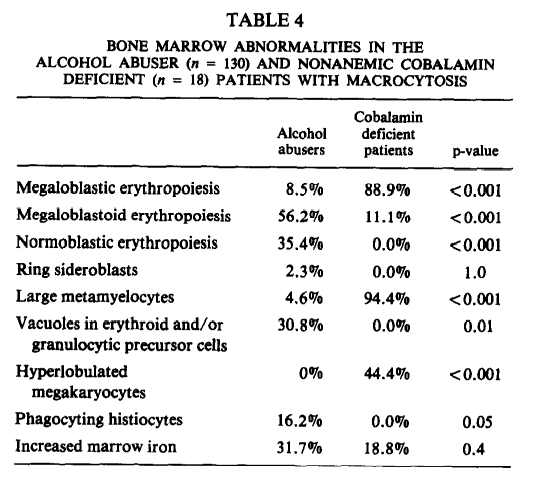
- Study of 18 nonanemic pernicious anemia patients and 136 alcohol abusers without deficiency of folate and with a mean cell volume (MCV) value > 100 fL. For purposes of this discussion we can focus on the group of patients with alcohol abuse.

Red blood cells
Bone marrow
- Vacuolation
- Numerous large vacuoles may appear in early red blood cell precursors.
- Vacuolation (vacuolization) of the red cell precursors was first described by McCurdy et al in 130 acutely intoxicated alcoholics:
- The degree of vacuolation was proportional to the amount of alcohol taken.
- Vacuolation was not associated with changes in circulating red cells.
- Similar, though less appreciable, changes were seen in granulocyte precursors.
- Vacuoles typically:
- Appear in the pronormoblasts 5 to 7 days following the initiation of heavy alcohol consumption.
- Disappear after 3 to 7 days of abstinence (often within 24 hours), although in some patients they persist for up to 2 weeks.
- Vacuolation occurs independently of megaloblastic and sideroblastic changes (they do not necessarily occur together).
- When experimentally induced by ethanol administration to human volunteers, vacuolation is dose-related.
- Ultrastructurally, the vacuolated nucleated red cells show marked membrane convolutions adjacent to the vacuoles, which lack organelles or organized structure, suggesting an effect of alcohol on the cell membrane.
- The functional significance of vacuolization is unknown. When produced experimentally, there is no associated decrease in hematocrit, reticulocyte count, or granulocyte count.
- Similar vacuolation seen with:
- Chloramphenicol administration
- Riboflavine or phenylalanine deficiency
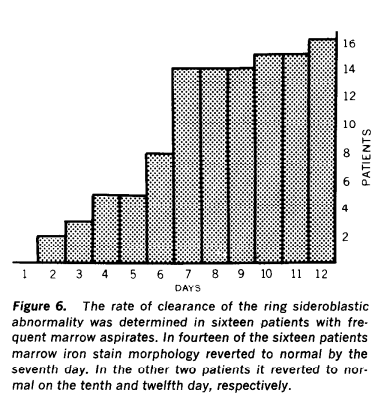
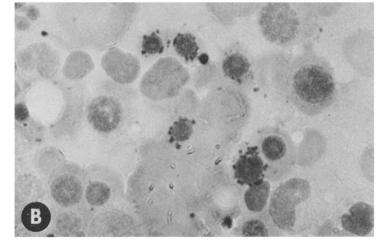
- Ringed sideroblasts
- First described in chronic alcoholics in 1964 by Hynes and Harris.
- Present in the bone marrow of about one-third of severe chronic alcoholics.
- In two series of consecutively studied patients, significant numbers of ring sideroblasts were found in the bone marrow aspirates of 29% and 31% of hospitalized alcoholics, respectively.
- Characterized by ferritin-rich granules that encircle the cell’s nucleus.
- Circulating red blood cells (RBCs) derived from ring sideroblasts contain granules rich in nonheme iron called Pappenheimer bodies, which may be detected on Wright’s-stained peripheral blood smears in one-third to one-half of cases.
- It is not known whether the presence of ring sideroblasts is associated with anemia, but patients may rarely develop a dimorphic blood picture, indistinguishable from that in severe sideroblastic anemia.
- The ringed sideroblasts generally disappear from the bone marrow within 5 to 10 days of abstinence, suggesting that they originate from a toxic effect of alcohol.
- Pathogenesis is not established. There are several possible explanations:
- Vitamin B6 deficiency (pyridoxal phosphate):
- Associated with reduced serum concentrations of pyridoxal phosphate; in one study:
- Treatment with pyridoxal phosphate, but not with pyridoxine and folic acid, caused reticulocytosis and disappearance of ring sideroblasts.
- This led to the conclusion that alcohol produces ring sideroblasts by interfering with conversion of pyridoxine to pyridoxal phosphate, thus impairing production of heme.
- However, initial reports of depression of erythrocyte pyridoxal kinase have not been confirmed by other investigators.
- Ethanol itself may directly inhibit heme synthesis in erythroid cells by inhibiting several enzymatic steps in heme synthesis.
- It is also possible that the characteristic iron deposition in the mitochondria of erythroid cells in ring sideroblasts is a nonspecific indicator of injury to this organelle by ethanol or acetaldehyde.
- Associated nutritional deficiency state that has not yet been clearly identified.
- Associated with reduced serum concentrations of pyridoxal phosphate; in one study:
- Vitamin B6 deficiency (pyridoxal phosphate):
- Megaloblastosis
- Megaloblasts occur frequently in the bone marrow of alcoholics.
- Many such patients have reduced folic acid levels in their red blood cells. Causes of folate deficiency include:
- Dietary deficiency (less common in current era of food fortification).
- Alcohol-mediated acceleration of the development of folic acid deficiency by:
- Reducing the absorption of folic acid from food.
- Suppression of hematopoietic response to folate:
- In one study, it was shown that:
- The intake of 15 ounces of alcohol daily could prevent or abort a reticulocyte response to physiologic amounts of folic acid (up to 75 pg daily) given orally or parenterally.
- This interference by alcohol could be overcome by increasing the dose of folic acid administered to 150 pg a day. When alcohol intake was discontinued, the administration of physiologic amounts of folic acid produced a normal reticulocyte response and a sharp decrease in serum iron level.
- In one study, it was shown that:
- May occur in absence or presence of anemia.
Peripheral blood
- Macrocytosis
- Macrocytosis in the absence of anemia is the most common hematologic change in the blood in alcoholics.
- Up to 80% of men and 46% of women with macrocytosis have been found to be alcoholics.
- Between 80% and 90% of patients taking more than 80 g ethanol daily (equivalent to 6 pints of beer, a bottle of wine or one third of a bottle of spirits) have macrocytosis.
- In one study, macrocytosis found in 4% of adult outpatients; the primary cause was chronic alcohol abuse in 65% of such patients.
- Red blood cell (RBC) size may correlate with the amount of reported drinking more strongly than the other conventional biochemical markers of ethanol consumption, such as gamma-glutamyltransferase.
- The degree of macrocytosis is typically modest with mean cell volume (MCV) no higher than 110 fL in the majority of patients.
- RBCs are typically uniformly round, in contrast to the more oval cells characteristic of megaloblastic anemia. Neutrophil hypersegmentation is absent.
- The macrocytes disappear and the MCV normalizes within 2 to 4 months of abstinence. The failure of the macrocytosis to improve during many weeks and months of abstention is not characteristic of the other “direct” effects of alcohol on hematopoiesis (such as megaloblastic and sideroblastic change, precursor cell vacuolization, thrombocytopenia).
- The precise mechanism underlying macrocytosis still is unknown. Not usually associated with folate deficiency or severe liver dysfunction.
- Anemia
- Anemia found in 13 to 62% of chronic alcoholics hospitalized for acute or chronic illness.
- Causes include:
- Megaloblastic changes in bone marrow:
- Uncommon cause of anemia in drinkers who are well-nourished.
- Serum and red cell folate concentrations may be low and serum cobalamin (vitamin BI2) levels are typically normal or elevated.
- In some patients (25 to 56%), folate levels are normal, but they respond to folate treatment.
- Prior to food fortification with folate, megaloblastic anemia developed more commonly in imbibers of wine and whiskey, which contain little or no folate, and is less frequently seen in those with a preference for beer, which is rich in the vitamin.
- In addition to causing folate deficiency, ethanol may have a direct toxic effect on marrow precursor cells, resulting in megaloblastic change.
- Sideroblastic changes in bone marrow.
- Hemolytic anemia:
- Stomatocyte hemolysis:
- Stomatocytes have a shortened life span because they become trapped in the small capillaries of the spleen and are subsequently destroyed.
- In healthy people, stomatocytes account for less than 5% of the RBC’s, whereas their number can be significantly higher in alcoholics. In fact, more than 25% of alcoholics exhibit an increased proportion of stomatocytes in the blood (i.e., stomatocytosis).
- The exact mechanism by which alcohol causes the formation of stomatocytes still is unclear.
- Zieve syndrome
- Hypophosphatemia, possibly via depletion of red cell adenosine triphosphate.
- Stomatocyte hemolysis:
- Alcohol withdrawal is often followed by a reticulocytosis, suggesting recovery from alcohol-induced bone marrow suppression. It remains an unproved possibility that alcohol suppresses erythropoiesis in the absence of megaloblastic or sideroblastic changes.
- In patients with alcoholic cirrhosis:
- Recurrent gastrointestinal hemorrhage and resulting iron deficiency
- Spur cell anemia
- Hypersplenism
- Inflammation (e.g., due to alcoholic hepatitis)
- Megaloblastic changes in bone marrow:
- In a prospective study of 121 alcoholic patients with anemia admitted to hospital, all of whom had a bone marrow biopsy:
- 45% had a single identifiable cause
- 37% had 2 apparent causes
- 18% had 3 or more causes
White blood cells
Because alcoholics commonly develop bacterial infections (including pneumococci, gram-negative bacilli, anaerobic organisms, Listeria, and the tubercle bacillus), much research has focused on alcohol’s effects on neutrophils, the primary cell of defense against bacterial invasion. However, alcohol also impairs the function of monocytes and macrophages, which attack bacteria and other microorganisms, and of lymphocytes, which mediate the immune response.
Neutrophils
Bone marrow
- Vacuolization in myeloid progenitor cells:
- Similar to that found in erythroid precursors but less frequent.
- Vacuoles are present in the cytoplasm and free of organized structure.
- The proportion of cells developing vacuoles appears correlating with the concentration of alcohol.
- In vitro culture of marrow cells from normal individuals in nutrient medium containing alcohol can induce cytoplasmic vacuolization.
- Chronic alcohol intoxication is associated with diminished marrow neutrophil reserves and neutropenia. Both in the presence and absence of infection, bone marrow cellularity is typically decreased, with neutrophil precursors arrested at early developmental stage (i.e., promyelocytes and myelocytes). The clinical data suggest that this may be a direct effect of ethanol, but experimental confirmation of this hypothesis in humans is lacking.
Peripheral blood
- Reduction of mature granulocytes:
- A striking feature of alcoholic patients with septic infection is that they often present with neutropenia. Paradoxical neutropenia typically occurs in alcoholics with severe bacterial infections, most commonly in association with pneumonia, and frequently accompanied by thrombocytopenia.
- The neutropenia, which is present on admission or develops shortly thereafter, is characteristically transient and is often followed by a “rebound” neutrophilia during the first hospital week if the patient survives.
- The decrease in circulating neutrophils is probably the result of a combination of diminished marrow reserves and increased cell utilization at the site of infection.
- Episodes of transient granulocytopenia also occur in association with alcohol intoxication in the absence of infection and have been reported in up to 8.5% of alcoholics, again with a high incidence of associated thrombocytopenia.
- Alcohol exposure also impairs functional activities of granulocytes:
- Alcohol consumption also interferes with the neutrophils’ ability to reach the site of an infection or inflammation (i.e., neutrophil delivery).
- Alcohol directly interferes with the capacity of neutrophils to adhere: in tissue-culture experiments, using nylon fibers to mimic this adherence, neutrophils could not adhere to the fibers if the blood samples were incubated with alcohol. This effect was more pronounced the higher the alcohol doses were.
- Indirect causes of neutropenia include:
- Hypersplenism
- Folate deficiency
- Infections
Lymphocytes
Chronic alcohol abuse may result in:
- Lymphopenia
- As severe in people who engage in a short period of binge drinking as it is in individuals who drank heavily for 6 months.
- Abstinence for 30 days was sufficient to restore lymphocyte numbers back to control levels.
- Reduced numbers of peripheral T cells
- Loss of naïve T cells, increase in memory T cells.
- Impaired T-cell activation both in humans and in mouse models.
- Impaired ability of T lymphocytes to migrate out of circulation.
Platelets
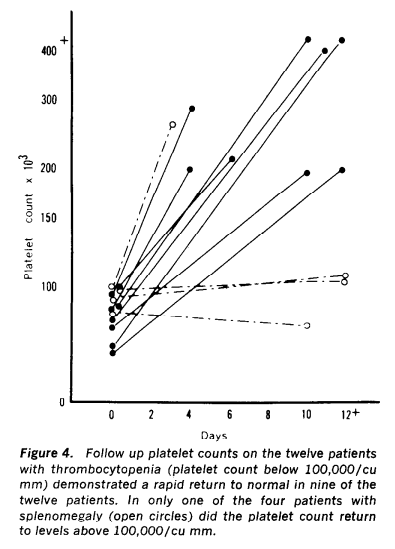
Thrombocytopenia (and rebound thrombocytosis)
- Bone marrow
- Alcohol-induced vacuolization may occur in the megakaryocytic lineage.
- Peripheral blood
- Sullivan and Herbert were the first to describe alcohol-induced thrombocytopenia in 1964.
- Prevalence of thrombocytopenia:
- 3%-43% of healthy, well-fed alcohol-dependent people
- 14%-81% of alcohol-dependent patients who require hospitalization
- 25% of hospitalized alcohol-dependent patients
- There is no relationship between the severity of thrombocytopenia and the degree of liver damage.
- Thrombocytopenia is rarely severe or life-threatening; if bleeding appears, other causes should always be considered.
- When ethanol is withdrawn, the platelet counts return from low to normal or supernormal levels (rebound thrombocytosis) in 1 to 3 weeks.
- Patients generally require no therapeutic intervention other than that needed to ease alcohol withdrawal.
- Thrombocytopenia often recurs with recurrence of heavy drinking.
- Possible pathogenic mechanisms of alcohol-associated thrombocytopenia include:
- Alcohol suppression of thrombopoiesis
- Alcohol-mediated degradation and apoptosis of platelets
- Thrombocytosis may also occur after withdrawal of alcohol in patients who are not thrombocytopenic at the time of admission.
Platelet function
In addition to affecting the size of platelets and causing thrombocytopenia, alcohol usually has a negative effect on platelet function. People with alcohol-related thrombocytopenia usually have longer bleeding times, which decrease as platelet normalizes.