Labs
Let’s begin with the patient’s complete blood count (CBC):
| WBC | Hb | MCV | PLT |
|---|---|---|---|
| 22.8 | 10.6 | 98 | 40 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45%, platelets (PLT) 150-450 x 109/L
The following is the patient’s white cell differential:

For mature white cells, it is advisable to focus on absolute counts rather than percentages because higher-than-normal or lower-than- normal counts are defined by thresholds in absolute numbers. This applies to those cell types that are enumerated by automated counters, namely neutrophils (which include segmented and band forms), lymphocytes, monocytes, eosinophils and basophils. To define which of the above counts are altered in our patient, we can refer to our “cheat sheet”:

For less mature forms, namely myelocytes, metamyelocytes and promyelocytes in the granulocyte lineage, it is common practice to interpret the percentages relative to the total white cell count, because these are assessed by manual counting and because the presence of even a small number of these cells is abnormal.
How is a left shift defined?
Additional definitions of left shift:
DynaMed:
Left shift indicates an abnormally high proportion of immature neutrophils in the blood, defined as either:
- Increased neutrophil bands in the peripheral blood (typically > 10% of white blood cells) by manual differential cell count
- Elevated I/T-G ratio* by manual differential cell count
- Elevated number or percent of immature granulocytes detected by automated cell analyzer, which typically represents promyelocytes, myelocytes, and metamyelocytes (but not blasts)
* Immature/total granulocyte (I/T-G) ratio defined as immature neutrophil count (myelocytes + metamyelocytes + bands +/- promyelocytes)/total neutrophil count; used as an indicator of a granulocyte left shift
Up-to-Date:
Left shift is an ill-defined term that refers to an increase in the percentage of band forms, generally accompanied by metamyelocytes and myelocytes.
Other definitions:
An increase in the ratio of nonsegmented/segmented neutrophils in the peripheral blood. J Clin Invest.1967;46:1943-1953.
A band/total neutrophil ratio or white blood cell count above 6% to 20% commonly used to indicate left shift. Clin Chim Acta. 2016 Jun 1;457:46-53.

What is a band neutrophil?
According to the College of American Pathologists:
- A band neutrophil is round to oval and 10 microns – 18 microns in diameter.
- The nuclear:cytoplasmic ratio is 1:1:5 to 1:2 and the nuclear chromatin is condensed.
- The nucleus is indented to more than half the distance to the farthest nuclear margin, but in no area is the chromatin condensed to a single filament.
- The nucleus can assume many shapes, including:
- Band-like
- Sausage-like
- S-shaped
- C-shaped
- U-shaped
- The cytoplasm contains primarily specific (pink) granules.
In addition to bands, the differential also showed increased numbers of metamyelocytes and myelocytes. These are less mature forms of granulocytes, situated further to the left on the differentiation schematic:
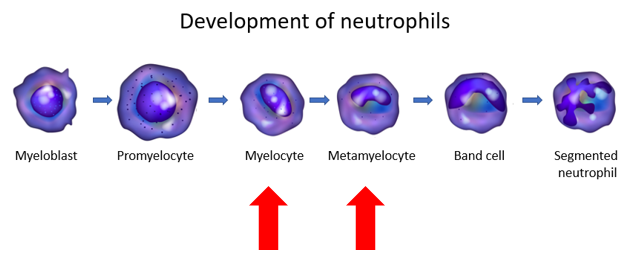

Does the patient meet the definition of leukemoid reaction?
Leukemoid reaction typically refers to a white blood cell count > 50-100 × 109//L that occurs in response to reactive causes.
Does the patient meet the definition of hyperleukocytosis?
Hyperleukocytosis typically refers to a white blood cell count > 100 × 109//L, usually found in leukemias and myeloproliferative disorders.
The patient’s differential showed neutrophilia, lymphopenia, and eosinopenia. This combination of changes is called a stress leukogram. It occurs in patients with stress of any kind, including in the setting of severe infection.
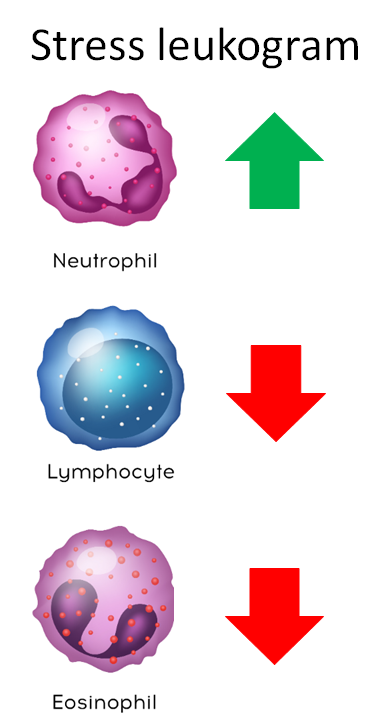

One way to remember this pattern is to consider that steroids (endogenous levels of which are increased in stress states) are used to treat lymphoma (by killing lymphocytes) and to treat hypereosinophilia (by killing eosinophils).
Monocyte counts tend to track with neutrophil counts in the stress response, but this is not always the case.
Based on what you know so far, your suspicion is highest for:
The patient’s platelet counts are low. What are possible causes of her thrombocytopenia?
What changes might you see in the following labs – decreased or increased values?
| Lab test | Result | Comment |
|---|---|---|
| Serum creatinine | ||
| Serum anion gap | ||
| Serum albumin | ||
| Serum ferritin | ||
| PT | ||
| aPTT | ||
| Plasma D-dimers | ||
| Serum TIBC | ||
| Serum lactate | ||
| Plasma fibrinogen |
What changes might you see in the following labs – decreased or increased values?
| Lab test | Result | Comment |
|---|---|---|
| Serum creatinine | Increased | If sepsis-associated acute kidney injury |
| Serum anion gap | Increased | If lactic acidosis from hypoperfusion |
| Serum albumin | Decreased | Negative acute phase reactant |
| Serum ferritin | Increased | Positive acute phase reactant |
| PT | Increased | May be elevated in DIC |
| aPTT | Increased | May be elevated in DIC |
| Plasma D-dimers | Increased | May be elevated in DIC |
| Serum TIBC | Decreased | Negative acute phase reactant |
| Serum lactate | Increased | If significant hypoperfusion from hypotension |
| Plasma fibrinogen | Decreased or increased | Low if DIC, high as positive acute phase reactant |
Here are the patient’s actual values (blue indicates increased, red indicates decreased):
| Lab test | Patient’s results (normal range) | Comment |
|---|---|---|
| Serum creatinine | 4.3 mg/dL (0.4-1.1 mg/dL) | Acute kidney injury |
| Serum anion gap | 26 mEq/L (10-18 mEq/L) | Her lactate was 10.9 mmol/L (see below) |
| Serum albumin | 3.1 g/dL (3.5-5.2 g/dL) | Negative acute phase reactant |
| Serum ferritin | 33,873 ng/ml (13-150 ng/ml) | Positive acute phase reactant |
| PT | 18.4 sec (9.4-12.5 sec) | Consistent with DIC |
| aPTT | 38.1 sec (25-36.5 sec) | Consistent with DIC |
| Plasma D-dimers | >21600 ng/ml (0-500 ng/ml) | Consistent with DIC |
| Serum TIBC | 204 ug/dL (260-470 ug/dL) | Negative acute phase reactant |
| Serum lactate | 10.9 mmol/L (0.5-2 mmol/L) | Hypotension |
| Plasma fibrinogen | 133 mg/dL (180-400 mg/dL) | Consistent with DIC, especially in context of her acute phase response |
Let’s summarize the labs:
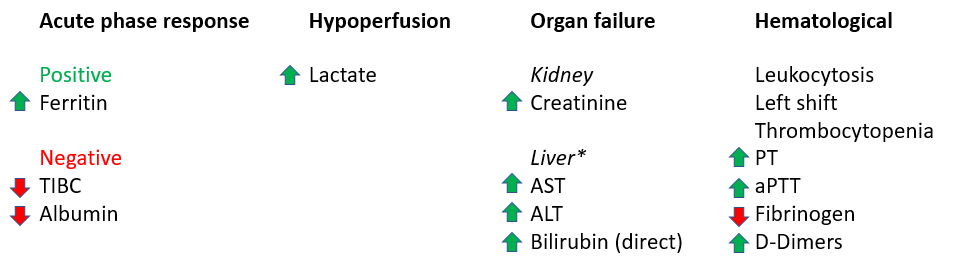

*To keep things simple, the patient’s liver function results are not shown, but they demonstrated evidence of shock liver with AST and ALT in the thousands IU, and a direct bilirubin that was was moderately elevated.
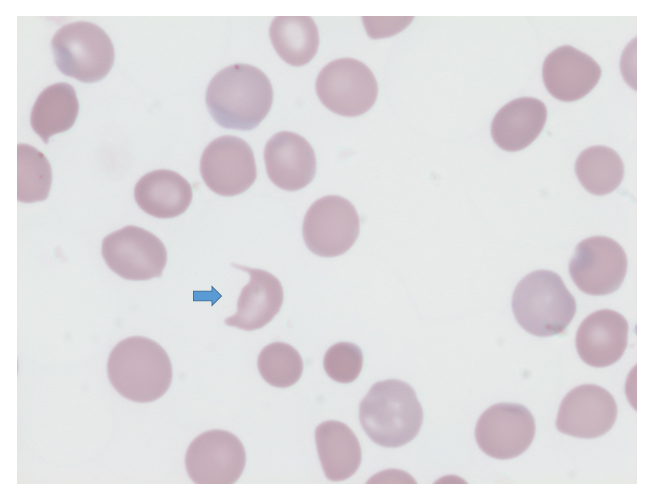
The patient’s peripheral smear shows presence of schistocytes, not unlike the following smear from a different patient.

How would you put together the hematological data?
The ferritin is extremely high. Does that mean the patient has hemophagocytic lymphohistiocytosis (HLH)?
So, you are concerned about disseminated intravascular coagulation (DIC). Is there a single test that is, by itself, capable of diagnosing DIC?
Should I use my gestalt or should I employ a clinical scoring system to make a diagnosis of disseminated intravascular coagulation (DIC) in a case such as this?
The following are recommendations from two independent practice guidelines regarding the use of clinical scoring system:
Recommendation from the Harmonization guideline:


Recommendation from the Japanese Society of Thrombosis and Hemostasis :


Bottom line: Use an established scoring system to make the diagnosis.
There are several clinical scoring systems for diagnosing disseminated intravascular coagulation (DIC). They use similar parameters, and each has been validated as a tool for diagnosing DIC and/or predicting poor outcome:
The ISTH subsequently published guidance based on harmonization of the recommendations from 3 of the guidelines.
ISTH, International Society of Thrombosis and Haemostasis; JMWH, Japanese Ministry of Health and Welfare; JAAM, e Japanese Association for Acute Medicine; JSTH, Japanese Society of Thrombosis and Hemostasis; SISET, Italian Society for Thrombosis and Hemostasis
The following table shows a comparison of the commonly used diagnostic scoring systems for disseminated intravascular coagulation (DIC):
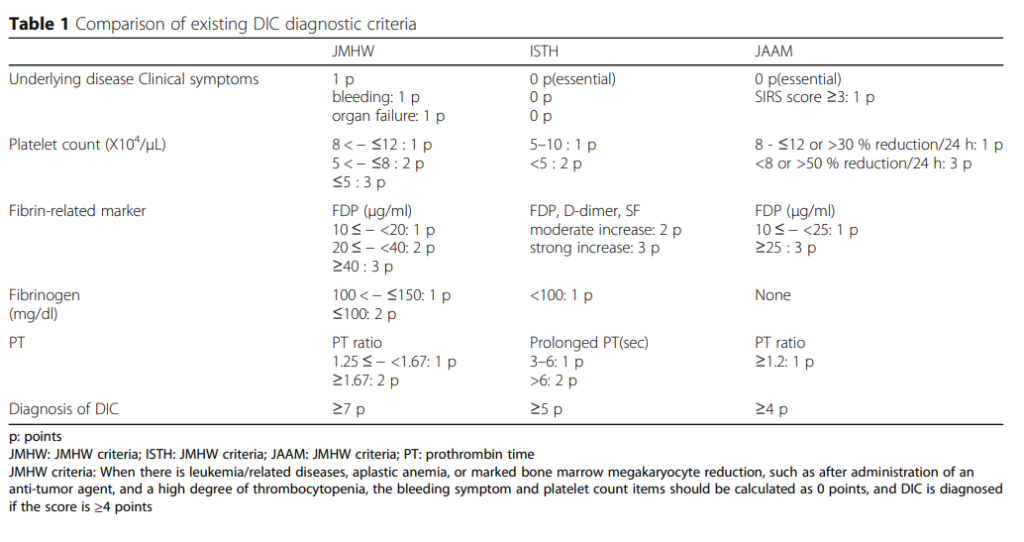

Note that for 2 of the 3 scoring systems (ISTH and JAAM), a diagnosis of DIC requires that patients have an underlying precipitating illness.
There are several common features and differences between these scoring systems:
Common features:
- All but one (JAAM) use the same 4 parameters:
- Platelet count
- Fibrin degradation products
- Fibrinogen
- Prothrombin time (PT)
Differences:
- Underlying disease is essential for ISTH and JAAM, but not for JMHW.
- Fibrinogen is graded for JMHW, but not ISTH. It is not included in the JAAM score.
- JAAM score takes into account the possibility that the platelet count is low in leukemia and other bone marrow disorders.
ISTH, International Society of Thrombosis and Haemostasis; JMWH, Japanese Ministry of Health and Welfare; JAAM, e Japanese Association for Acute Medicine; JSTH, Japanese Society of Thrombosis and Hemostasis; SISET, Italian Society for Thrombosis and Hemostasis
Let’s look at the 4 parameters in turn.
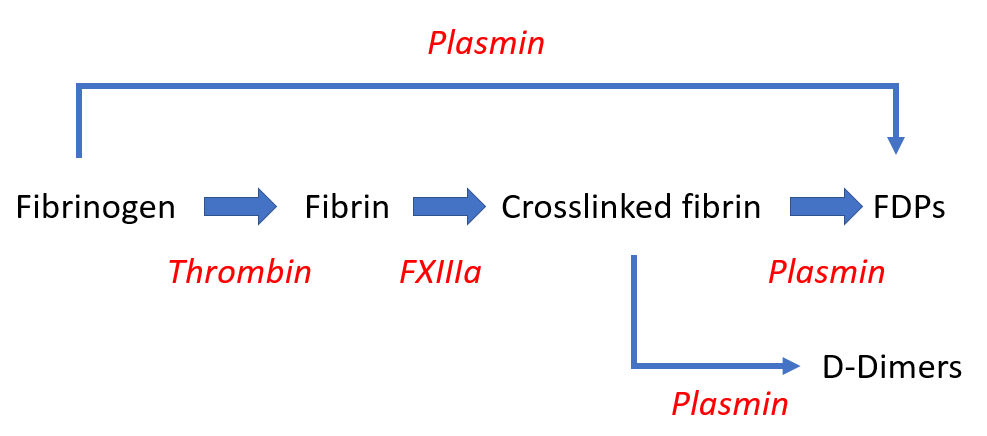
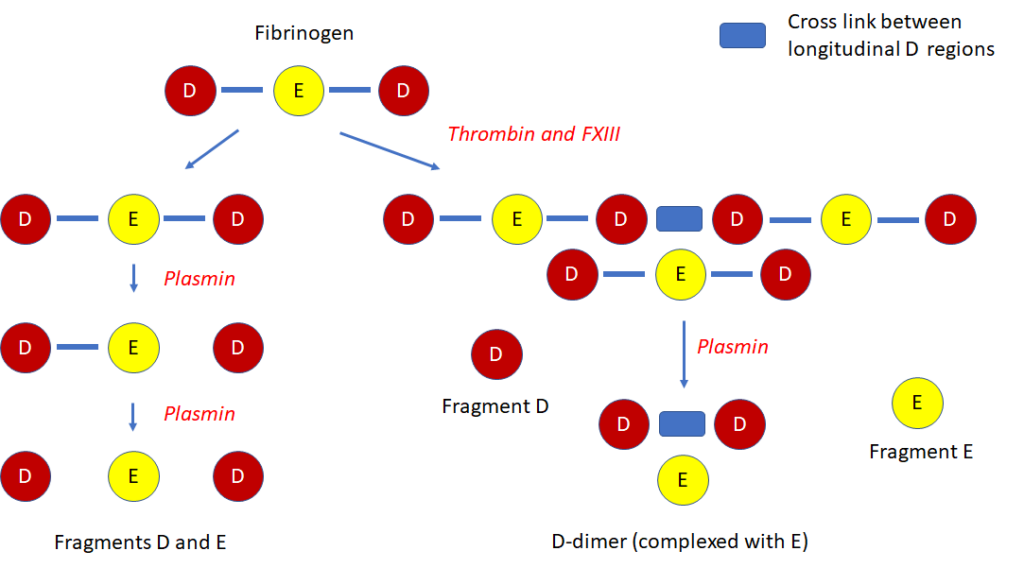
Fibrin-fibrinogen degradation products (FDPs), D-dimer
High sensitivity, low specificity (may be increased in cases of deep vein thrombosis, pulmonary thromboembolism, massive hydrothorax/ascites, and large subcutaneous hematomas).
D-dimer:
- A degradation product of cross-linked fibrin.
- The smallest circulating FDP that is specific for fibrinolysis.
- Contains two cross-linked D fragments of the fibrin protein, hence its name.
- Quantified by immunoassay.
- Elevated in patients with disseminated intravascular coagulation (DIC), but high levels can also be found in patients with venous thromboembolism, recent surgery, or inflammatory conditions.
FDPs:
- FDP assays measure the breakdown split products of either fibrinogen or fibrin.
- Increased values indicate enhanced fibrinogenolysis or fibrinolysis.
- Quantified by immunoassay.
- Lacks specificity because in addition to DIC, high levels can be found with trauma, recent surgery, inflammation, venous thromboembolism, and many other conditions.
- Because FDPs are metabolized in the liver and cleared by the kidneys, levels are influenced by liver and kidney function.


Thrombocytopenia:
- High sensitivity, low specificity for diagnosis of disseminated intravascular coagulation (DIC).
- Reported to occur in up to 98% of DIC cases.
- Most patients with DIC have platelet counts < 100 × 109/L.
- Platelet count < 50 × 109/L reported to occur in 10%-50% cases.
- Low counts correlate with thrombin generation.
Fibrinogen:
- Levels may decrease owing to consumption (increased conversion of fibrinogen to fibrin).
- Levels may be further decreased when there is predominant hyperfibrinolysis (which can result in direct cleavage of fibrinogen).
- Fibrinogen is an acute phase reactant, thus plasma levels may remain in normal range despite ongoing consumption.
- Low fibrinogen level has high specificity, but low sensitivity for disseminated intravascular coagulation (DIC).
- Overall sensitivity of low fibrinogen level reported to be only 28%, with hypofibrinogenemia occurring only in very severe cases of DIC.
- Not a diagnostic criteria in the JAAM score.
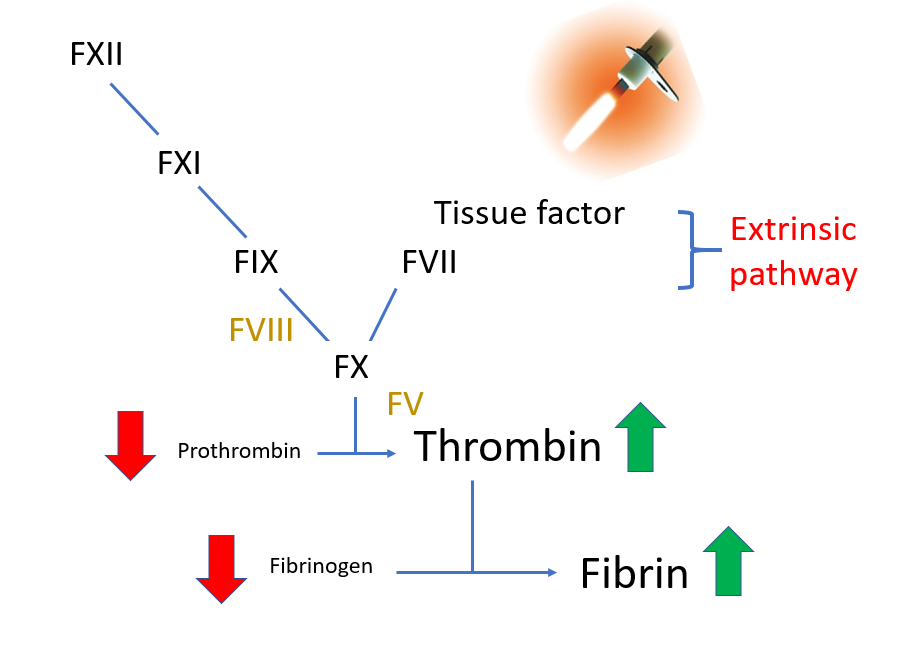


Prothrombin time (PT):
- Increased in about one-half patients with disseminated intravascular coagulation (DIC) at some point during the course of the disease.
- More sensitive than aPTT, which may be normal due to increased levels of factor VIII (FVIII), an acute phase reactant.
- Not specific for DIC; elevated PT also occurs in patients with congenital factor VII deficiency, liver disease and vitamin K deficiency.
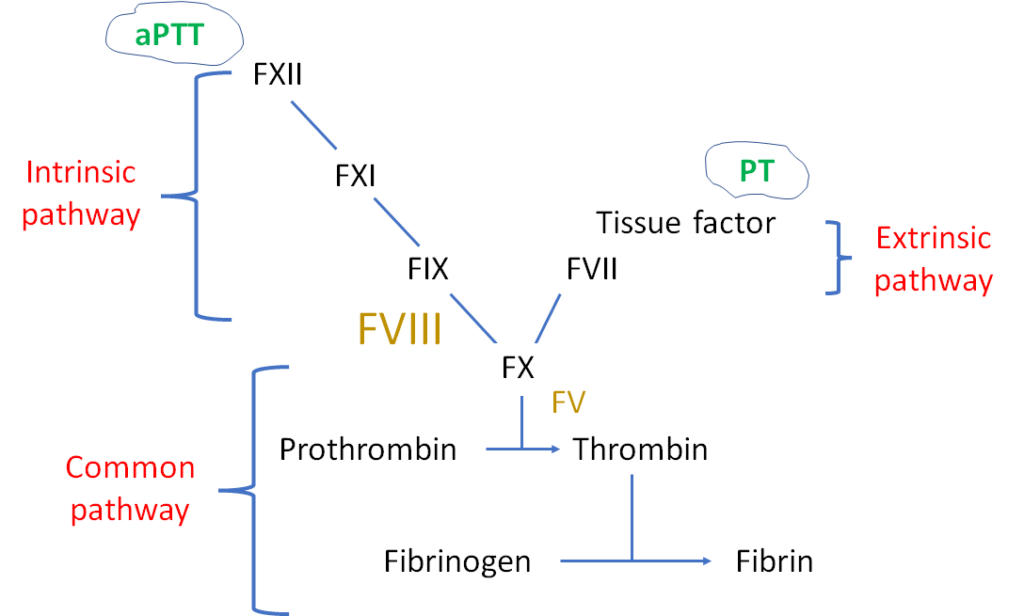

Recall that most guidelines require an underlying cause of disseminated intravascular coagulation (DIC). Here are some potential etiologies to look for:




The ISTH scoring system is simple! It consists of just 4 parameters based on widely available, routine coagulation tests.
| Parameter | Score | Comment |
|---|---|---|
| Fibrinogen (g/L) | >1 (0) <1 (1) | Overall sensitivity of low fibrinogen level reported to be about 30%. |
| Prothrombin time (PT) (seconds) | <3 (0) 3-6 (+1) >6 (+2) | Elevated in 50%-60% of patients with DIC at some point during course of illness. |
| Platelet count (109/L) | >100 (0) 50-100 (+1) <50 (+2) | Thrombocytopenia reported to occur in up to 98% of DIC cases. |
| D-dimers or FDPs | No increase (0) Moderate increase (+2) Severe increase (+3) | Elevated FDPs and D-dimers are sensitive, but not specific for DIC. |
Data are presented as values with number of points in parentheses.
A total score of ≥5 is compatible with diagnosis of DIC.
Let’s apply the ISTH scoring system to our patient (right-most column):
| Parameter | Score | Patient’s results (points) |
|---|---|---|
| Fibrinogen (g/L) | >1 (0) <1 (1) | 1.3 (0) |
| Prothrombin time (PT) (seconds) | <3 (0) 3-6 (+1) >6 (+2) | 7 (2) |
| Platelet count (109/L) | >100 (0) 50-100 (+1) <50 (+2) | 40 (2) |
| D-dimers or FDPs | No increase (0) Moderate increase (+2) Severe increase (+3) | Severe increase (3) |
| Total score | 7 |
Data are presented as values with number of points in parentheses.
A total score of ≥5 is compatible with diagnosis of disseminated intravascular coagulation (DIC).
This patient’s score of 7 is diagnostic of DIC.
To reiterate, DIC is diagnosed when the ISTH DIC score is 5 or higher:
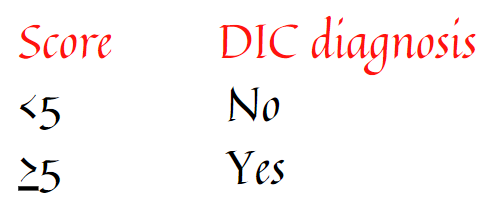
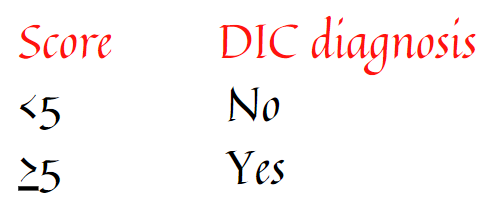
Learn more about the ISTH DIC score:
- Original publication on the ISTH score can be found here.
- Prospective validation study can be found here – sensitivity of the DIC score 95%, specificity 98%.
- See ISTH calculator.
To summarize the testing section:
- The patient’s clinical presentation together with leukocytosis, stress leukogram, and left shift is consistent with infection.
- The ISTH disseminated intravascular coagulation (DIC) scoring system is consistent with a diagnosis of DIC.
- Likely diagnosis is DIC secondary to severe infection.

