Labs
The following is the patient’s complete blood count (CBC) at the time of admission:
| WBC (109/L) | Hb (g/dL) | MCV (fL) | PLT (109/L) |
|---|---|---|---|
| 6.8 | 13.6 | 87 | 398 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45 fL, platelets (PLT) 150-450 x 109/L
| PT (seconds) | aPTT (seconds) |
|---|---|
| 12.5 | 45 |
Thus, there is an isolated elevation in the aPTT.
What tube is blood collected in for measuring the PT and aPTT?
Let’s look at how a PT and aPTT are carried out:
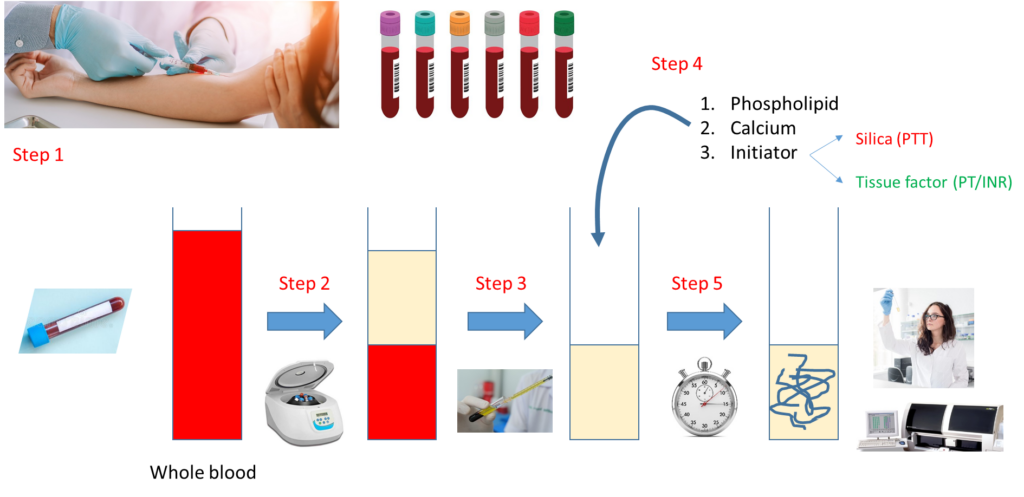
Notes:
- Step 1 – Draw whole blood from a blood vessel (typically an arm vein) into a blue top tube containing the anticoagulant, sodium citrate.
- Step 2 – Spin the liquid blood sample in a centrifuge so that red cells layer on the bottom, plasma on the top.
- Step 3 – Pipette plasma (top layer) into a clean, empty test tube.
- Step 4 – Add phospholipid, calcium and activator (tissue factor for PT, silica for aPTT).
- Step 5 – Incubate at 37 degrees C and measure time to clot formation, either with automated instrument or more rarely by vision.
Note that the major difference between the PT and aPTT is the nature of the activator added to the sample.
What are possible causes of an isolated elevation in the aPTT (i.e., the PT is normal)?
Further work-up of this case requires some knowledge of the clotting cascade. Let’s review the basics:
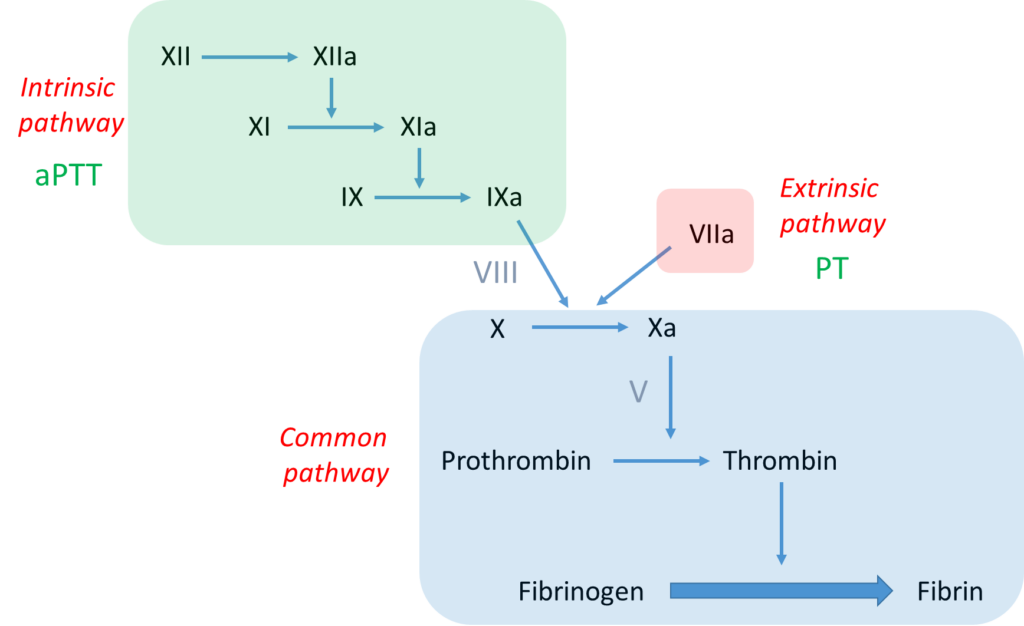
Notes:
- The bottom line is formation of an insoluble fibrin plug, which, along with platelets, stems blood loss.
- Fibrin is derived from thrombin-mediated cleavage of soluble fibrinogen (which is a structural protein).
- Thrombin (which is a type of enzyme called a serine protease) is formed when another serine protease, activated factor X (FXa), cleaves prothrombin.
- Two pathways may activate factor X:
- The intrinsic pathway, which consists of a series of linked reactions involving serine proteases FXII, FXI and FIX, and cofactor FVIII.
- The extrinsic pathway, which consists of tissue factor-mediated activation of FVII (FVIIa).
- In vivo, the clotting cascade is always initiated by tissue factor activation of FVII (extrinsic pathway) and amplified by the intrinsic pathway via cross-talk (FVIIa activates FIX) and feedback (thrombin activates FXI and FVIII) mechanisms.
- aPTT measures the integrity of the intrinsic pathway.
- PT/INR measures the integrity of the extrinsic pathway.

According to the scheme above, an isolated elevation in the aPTT indicates a deficiency of or inhibitor against a clotting factor in the intrinsic pathway, namely FXII, FXI, FIX or FVIII.
What would you like to test for next?
A mixing study is a reasonable next step in a patient with unexplained elevation in PT and/or aPTT. What is a mixing study?
Let’s review the basics of the mixing study:
Notes:
- Mixing study involves mixing patient plasma and pooled normal plasma in 1:1 ratio.
- The resulting mixture is then processed for clotting time (PT or aPTT) immediately (time 0) and then after 2 hours of incubation.
- A clotting factor must drop below about 30% before the PT or PTT begins to prolong.
- Therefore, even if a clotting factor is completely missing (i.e. 0% level), the 1:1 mix will restore it to 50% (equal volume of plasma containing 0% of the factor and normal pooled plasma containing 100% of the factor), more than enough to restore a normal PT or aPTT (the example above shows 10% activity of imaginary factor Y in patient plasma, with 1:1 mix resulting in 60% activity).
- However, if there is an inhibitor or antibody to a clotting factor, the inhibitor will carry over from patient to normal plasma in the mix and interfere with factor activity, leading to continued prolongation of the PT or aPTT.
Mixing study (50:50 mix) results in our patient:
(baseline aPTT 43 seconds)
aPTT
31.2 seconds at time 0
35 seconds at 2 hours
What is the result of the above mixing study?
To summarize at this point, we have a patient with excessive postoperative bleeding and a prolonged aPTT that corrects with normal plasma (a negative inhibitor screen or normal mixing study). These results indicate deficiency of one or more clotting factors rather than an inhibitor against a clotting factor.
At this point what would you check first?
Factor VIII level was 26% (normal 50-150%).
What are possible diagnoses?
AVWS, acquired von Willebrand syndrome
Results of the von Willebrand (vWF) screen:
vWF antigen 29% (normal 60-158%)
vWF activity < 12.5% (normal 50-200%)
About von Willebrand factor (vWF) screening tests:
The antigen test is an immunoassay that measures the concentration of vWF protein in plasma.
The activity assay is also called von Willebrand factor ristocetin cofactor (vWF:RCo) activity. It is a functional assay that measures the ability of ristocetin to promote platelet aggregation in the presence of vWF.

Laboratory findings in acquired von Willebrand syndrome (AVWS) are similar to those in von Willebrand disease (VWD) and may include decreased values for VWF:Ag, VWF:RCo, or FVIII. The VWF multimer distribution, which can be ordered as a separate test often shows a decrease in large multimers similar to the pattern seen in type 2A VWD.
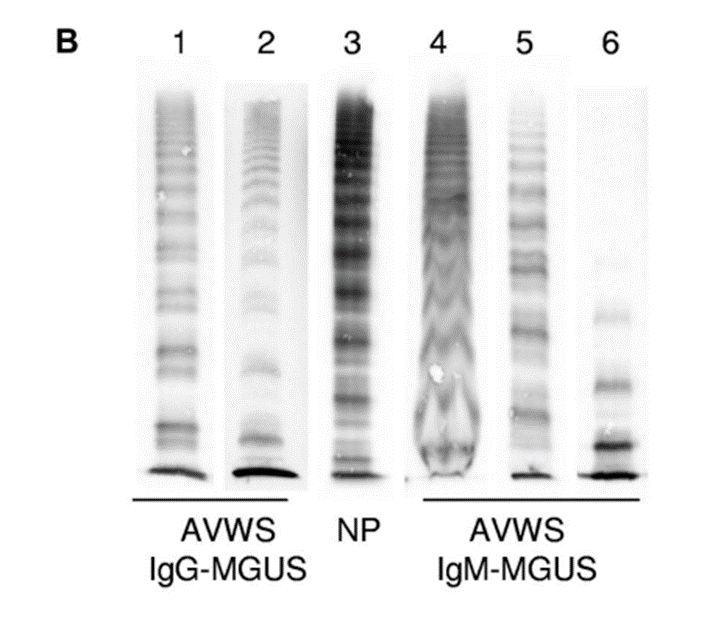

In this case, the vWF antigen level was low, and the vWF activity even lower. In other words, the vWF:RCo/vWF:Ag ratio is reduced. A low vWF:RCo/vWF:Ag ratio is seen in hereditary type 2A VWD where there is a reduction in high molecular weigh VWF multimers. It is also low in many patients with acquired VWS, because they are too are deficient in high molecular weight vWF multimers.

What are important considerations in patients with acquired VWS (AVWS)?
This patient had excessive bleeding around the time of his hernia repair. However, that has resolved without requiring any hemostatic treatment. He is stable and ready to be discharged home.
Examples of potential work-up for the causes of acquired von Willebrand syndrome (AVWS), as guided by the clinical context:
| Cause | Examples | Tests |
|---|---|---|
| Lymphoproliferative or plasma cell disorders | MGUS, MM, WM, lymphoma | CBC, SPEP |
| Myeloproliferative neoplasms | Polycythemia vera, essential thrombocythemia | CBC |
| Cardiac disease | Aortic stenosis, hypertrophic cardiomyopathy | Echocardiogram (if indicated) |
| Circulatory assist devices | ECMO, LVAD | N/A |
| Medications | Ciprofloxacin, griseofulvin, valproic acid | N/A |
| Autoimmune disorder | SLE | ANA |
| Sold tumor | Wilms | CT scan (if indicated) |
| Hypothyroidism | Of any cause | TSH |
This patient had an SPEP. The result was reviewed by the hematologist on a follow-up clinic visit:
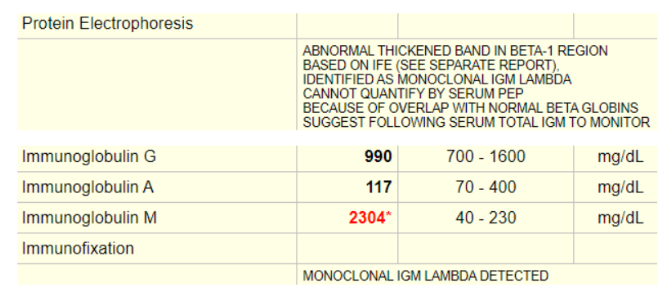
In summary, the patient has a monoclonal IgM gammopathy as defined by serum protein electrophoresis (SPEP) in combination with immune fixation. The differential diagnosis of monoclonal IgM gammopathy includes IgM-MGUS (monoclonal gammopathy of unclear significance), Waldenström’s macroglobulinemia (WM), and IgM myeloma.
- IgM MGUS is defined by an IgM serum protein of less than 3 g/dL, less than 10% clonal lymphoplasmacytic cells in the bone marrow, and the absence of symptoms typical of WM.
- WM is defined by a monoclonal IgM serum protein and at least 10% monoclonal lymphoplasmacytic cells in the bone marrow clot section or biopsy.
A bone marrow aspirate and biopsy were performed:
Aspirate Smear: The aspirate material is adequate for evaluation, and consists of multiple cellular spicules. The M:E ratio is 2:1. Erythroid precursors are normal in number and exhibit normoblastic maturation. Myeloid precursors are normal in number and show normal maturation. Megakaryocytes are normal in number. Abnormal forms are not seen. Plasma cells are seen singly and in small aggregates. A 500 cell manual differential shows: 1% blasts, 5% promyelocytes, 3% myelocytes, 8% metamyelocytes, 34% bands/neutrophils, 18% eosinophils, 23% erythroids, 22% lymphocytes, 7% plasma cells, 2% basophils.
Clot Section and Biopsy Slides: The core biopsy material is adequate for evaluation. It consists of a 0.5 cm core biopsy of trabecular marrow with a cellularity of 30-40%. The M:E ratio estimate is normal. There is an interstitial infiltrate of mononuclear cells consistent with plasma cells occupying 10% of overall cellularity. Erythroid precursors are normal in number and have overall normoblastic maturation. Myeloid precursors are normal in number with normal maturation. Megakaryocytes are normal in number with focal tight clustering. A homogenous fibrillary material is present, which is favored to be collagen; negative for Amyloid by stains.
Immunophenotype profile: positive for surface IgM, CD19, CD20, and CD22.
Note: WM is defined by plasma cell component of overall cellularity on clot section or core biopsy, not bone marrow aspirate.
Based on this information the patient was diagnosed with Waldenstrom macroglobulinemia (WM), whose definitive diagnosis requires monoclonal immunoglobulin M (IgM) gammopathy and evidence of bone marrow infiltration by lymphoplasmacytic cells supported by immunophenotypic studies, using either flow cytometry or immunohistochemistry.
Given the known association between WM and acquired von Willebrand syndrome (AVWS), it was felt that the Waldenstrom macroglobulinemia (WM) accounted for the AVWS.
As noted in the history, the patient has a history of recurrent iron deficiency anemia.
Assuming that this represents blood loss and not malabsorption of iron, what is the most likely source of blood loss?
Gastrointestinal blood loss in a patient with acquired von Willebrand syndrome (AVWS) raises a suspicion for what condition?

