About the Condition
Description/definition:
Factor V (FV) deficiency is a rare bleeding disorder, which may be due to homozygous or compound heterozygous mutations of the FV gene or less commonly to an acquired inhibitor:
- Congenital FV deficiency affects an estimated 1 in 1,000,000 individuals and represents 9% of rare bleeding disorders. It is most often diagnosed in childhood.
- Acquired FV inhibitor has an estimated incidence <0.5 per million person years. It usually occurs after the sixth decade of life (see report).
Classification of FV deficiency:
1. Congenital
2. Acquired:
- Inhibitors:
- Primary or idiopathic – approximately 20% of patients with acquired factor V inhibitors are idiopathic.
- Secondary:
- Drugs – most common cause:
- Antibiotics, mainly beta-lactams — including:
- Third-generation cephalosporins
- Penicillin
- Occurs at a median of 16 days after treatment initiation.
- Exposure to bovine protein – Factor V inhibitors may be associated with surgical procedures in which topical bovine thrombin is used (also contains bovine FV).
- Antibiotics, mainly beta-lactams — including:
- Malignancy, including multiple myeloma
- Infection
- Autoimmune disease
- Transfusion of fresh frozen plasma in patients with severe congenital FV deficiency
- Drugs – most common cause:
- Increased non-immune clearance – amyloidosis

Pathophysiology:
FV is a 330-kDa glycoprotein produced in the liver that circulates in plasma. Approximately 20% of FV is stored in the alpha granules of platelets and can be released at sites of vascular injury with platelet degranulation.
Congenital deficiency:
- The F5 gene is located on chromosome 1q24.2 consisting of 25 exons.
- Missense, nonsense, splicing, and insertion/deletion mutations have been reported among 26 F5 variants identified in FV-deficient patients.
Acquired deficiency:
- Usually associated with one of the following:
- Spontaneous autoantibodies – these often increase clearance of FV rather than neutralize FV activity (see report). As with the patient in this case study, the test results for factor V inhibitors may be negative if a patient has clearance-facilitating antibodies.
- Antibodies cross-reacting with anti-bovine factor V.
- Alloantibodies (cases where fresh frozen plasma is transfused into patients with severe congenital deficiency of FV).

Diagnosis:
Consider the diagnosis of FV deficiency in a patient with:
- Congenital deficiency:
- Bleeding early in life
- Elevated PT and aPTT
- Acquired deficiency:
- Recent onset of bleeding symptoms, especially in older adults.
- Lack of previous bleeding symptoms especially in association with previous hemostatic challenges.
- No family history of congenital/inherited deficiency of FV (nor other coagulation factor deficiencies).
- Elevated PT and aPTT
Confirm diagnosis:
- Congenital deficiency:
- Correction of prolonged PT and aPTT by normal plasma (mixing study).
- Reduced factor V activity in specific factor assay.
- Correlation between factor V activity and severity of disease manifestation is weak.
- Acquired deficiency:
- If inhibitor, no correction of prolonged PT and aPTT with normal plasma.
- If deficiency, correction of prolonged PT and aPTT with normal plasma (mixing study); however, as seen in this case, if there is an antibody that increases clearance of FV rather than neutralizing its activity, the prolonged PT and aPTT may correct with normal plasma in the mixing study.
- Reduced factor V activity in specific factor assay.
Other labs:
- Factor V antigen assay performed by immunoassay can help distinguish between quantitative and qualitative factor V deficiency.

Treatment:
General principles of management:
- Treatment of active bleeding
- Eradication of inhibitor
- Treatment of underlying condition
Treatment of active bleeding (hemostatic treatment):
Note: To date, there are no available commercial FV concentrates.
Fresh frozen plasma (FFP):
- Administered as replacement therapy (for treatment or prevention of bleeding).
- Consider FFP 15-25 mL/kg with additional FFP 10 mL/kg at 12-hour intervals if needed.
- Contains only 1 U/mL of FV and is easily overwhelmed by FV neutralizing autoantibodies in patients with acquired FV deficiency.
- Risk of circulatory overload.
Platelet transfusion:
- Platelets are an alternate source of FV; they contain 20% of plasma FV.
- May be used to supplement FFP, adjusted to maintain factor V activity at > 15%-20%.
Antifibrinolytics:
- In patients with mild to moderate factor V deficiency, treatment with antifibrinolytics is usually sufficient to control epistaxis, menorrhagia, or other non-life-threatening mucosal bleeding.
- Contraindicated in patients with urinary tract bleeding.
- Tranexamic acid 15-20 mg/kg or 1 g 4 times daily alone.
Recombinant FVIIa or prothrombin complex concentrates (PCCs):
- Only used when all other therapeutic modalities fail to control bleeding adequately.
- Use of recombinant factor VIIa reported in patients allergic to FFP, those with factor V inhibitors, or those who wish to avoid volume overload.
Eradication of inhibitor (in cases of acquired FV deficiency):
- The following have been used in patients with immune-mediated acquired FV deficiency:
- Pulse prednisolone alone
- Prednisone plus cyclophosphamide or rituximab
- Plasma exchange
- High-dose intravenous immunoglobulin (IVIG)
Treatment of underlying conditions:
- Stop any inciting drug.
- Treat underlying condition such as multiple myeloma or amyloidosis.
Similar study:
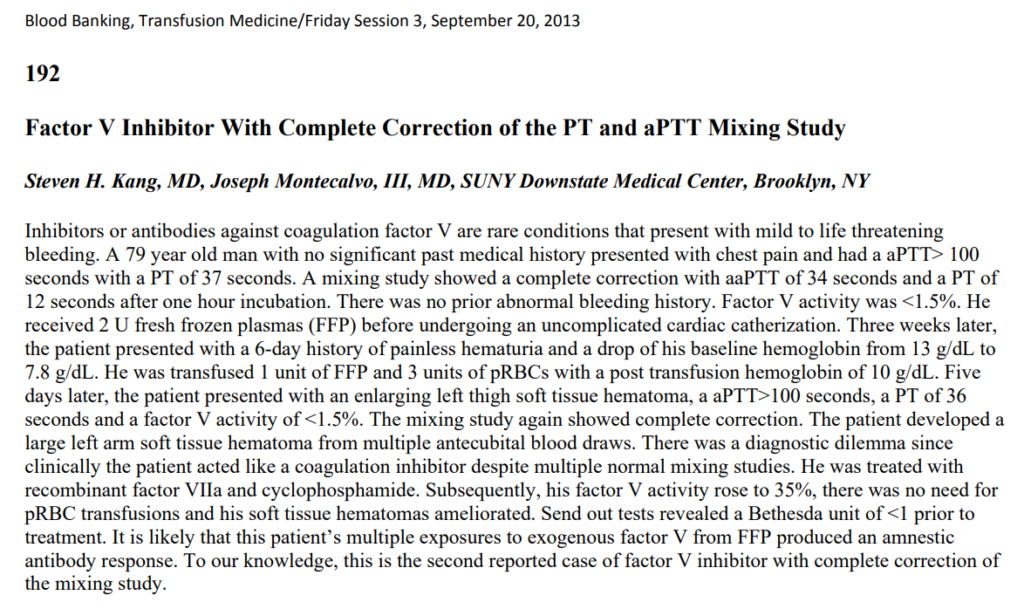
Also see report.

