Labs
You order a complete blood count (CBC), but the hematology lab reports back that the patient’s blood sample cannot be read by the automated hematology analyzer. They suggest you resend blood and keep the sample at 37 degrees F during transport to the lab. You make arrangements to send such a sample, and the CBC is successfully completed (next slide).
For more information on how cold agglutinins interfere with the CBC, click here.
The following is the original complete blood count (CBC) on blood at room temperature (1) and warmed blood (2):

Let’s reorganize the CBC on the warmed blood sample according to our trusted TBP format:
| WBC (109/L) | Hb (g/dL) | Hct (%) | MCV (fL) | RDW-SD (fL) | PLT (109/L) |
|---|---|---|---|---|---|
| 5.3 | 8.0 | 27.2 | 78 | 52 | 234 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45 fL, platelets (PLT) 150-450 x 109/L
A question for the more experienced:
For more information on how cold agglutinins interfere with the CBC, click here.

What is the first lab test (other than peripheral smear) we should obtain in a patient with normocytic anemia?
Click for AnswerThe reticulocyte count was 5%. Is this an appropriate reticulocyte count in the setting of anemia? (we may want to change the number to higher)
Another way to determine whether the reticulocyte count is appropriate or not is to consider the absolute reticulocyte count. This is provided by some labs. In other cases, it can be calculated from the red cell count and the % reticulocytes (maybe include in the link out to retics on previous slide).
For example:
| Reticulocyte % | Red blood cell count | Absolute reticulocyte count |
|---|---|---|
| 1% | 5 x 1012/L | 50 x 109/L |
| 5% | 5 x 1012/L | 250 x 109/L |
| 10% | 5 x 1012/L | 500 x 109/L |
An absolute reticulocyte count of > 120 x is considered an appropriate response to anemia!
So, the patient appears on the “appropriate reticulocyte count” side of the diagnostic ledger:
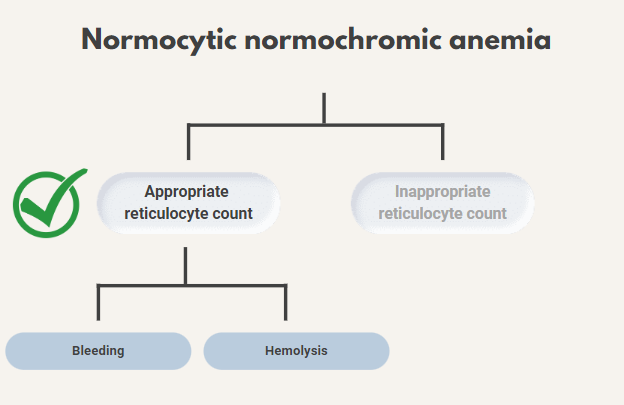
He shows no signs of bleeding, so you decide to focus on the possibility of hemolytic anemia.

Sort the lab parameters according to their expected blood levels in hemolysis?
A few hours later, his hemolysis labs return [please provide normal ranges, we can then make a table]:
- AST 25
- ALT 30
- ALP 170
- LDH 300
- Tbili 2.5 [indirect should be high!]
- Dbili 2.0
- Haptoglobin <8
True or false: the mean cell volume (MCV) is always normal in a patient with elevated reticulocytes.
Thus far, the data support a diagnosis of hemolytic anemia. Before we consider the peripheral smear, let’s discuss an approach to hemolysis.
What is hemolytic anemia?
In normal physiology, red cells last approximately 120 days in circulation. As they circulate, they gradually develop wear-and-tear damage as they pass through small capillaries or across cardiac valves, as well as oxidative damage to their membranes. Eventually, this damage makes them less flexible. They become unable to navigate the narrow splenic sinuses, and are eventually phagocytosed by splenic macrophages.
“Hemolysis” is an umbrella term that refers to the premature destruction of red blood cells – they are destroyed before reaching the point of physiologic, senescent death. There are a wide variety of processes that can lead to hemolysis, which we will explore below.
What can cause hemolysis?
Key point: there are a wide variety of causes. It helps to have a systematic approach.
What are some approaches for generating a differential diagnosis?
1. Mnemonic-associated approaches can be useful, especially for broad differentials. A popular mnemonic is shown below:
| V | Vascular |
| I | Infectious/Inflammatory |
| N | Neoplastic |
| D | Drugs/Deficiency |
| I | Idiopathic/Iatrogenic |
| C | Congenital |
| A | Autoimmune |
| T | Traumatic |
| E | Endocrine/Metabolic |
Mnemonics can help ensure you maintain a broad differential, without missing a key group of etiologies. However, mnemonics are nonspecific. Sometimes it can be helpful to consider a physiologic or anatomic approach to formulating your differential.
2. A diagnostic schema offers a systematic approach to generating a differential diagnosis for a particular problem. Schemas generally subdivide your differential into various physiologic “buckets”. We will explore a diagnostic schema for hemolysis on the next slide.
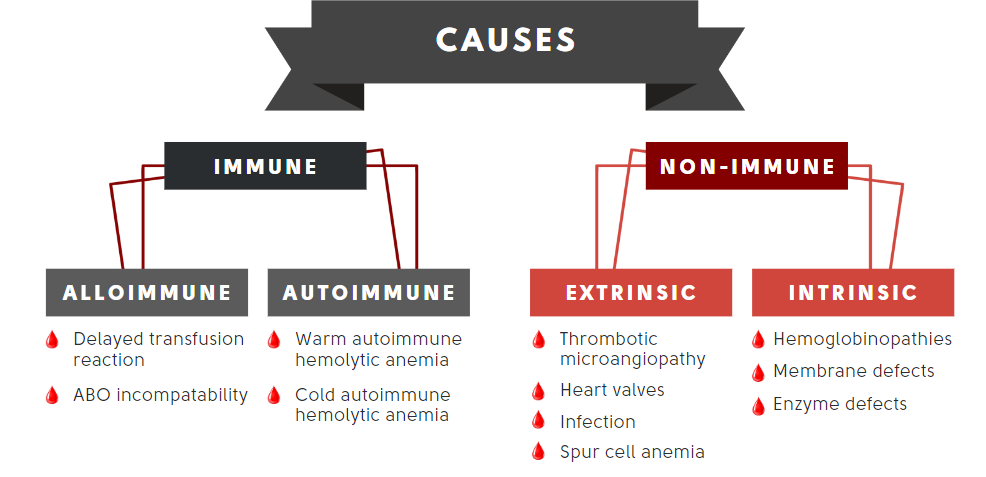
Let’s organize the differential diagnosis of hemolytic anemia in table format and consider appropriate next steps, including a peripheral smear, blood work and imaging:
| Smear (in addition to polychromatophilia) | Other tests | Comments | |
|---|---|---|---|
| Immune-mediated hemolysis | Spherocytes, agglutination | Direct antiglobulin test | This cannot be ruled out in our patient |
| Non-immune | |||
| Extrinsic | |||
| TMA | Schistocytes, thrombocytopenia | Renal function, ADAMTS13 | Typically presents with acute illness and low platelet count |
| Spur cell anemia | Acanthocytes, target cells | Liver function tests, liver imaging | No evidence of liver disease |
| Infection | Smear positive for parasites | Blood cultures (clostridium) | No recent travel, no known exposure to ticks |
| MAHA | Schistocytes | Valve imaging | No recent valve repair or replacement, not a marathon runner, does not play drums with hands |
| Intrinsic | |||
| Sickle cell disease | Sickle cells, nucleated RBCs, Howell-Jolly bodies | Hb electrophoresis | Not the right demographics |
| Thalassemia | Microcytic red blood cells, target cells | Hb electrophoresis, DNA sequencing | Cannot rule out |
| Hereditary spherocytosis | Spherocytes | Osmotic fragility test/EMA binding test | Cannot rule out. Patients can present in adulthood |
| Heredity elliptocytosis | Elliptocytes | Osmotic gradient ektacytometry | Cannot rule out. Patients can present in adulthood |
| Paroxysmal nocturnal hemoglobinuria | Often normal | Flow cytometry | Cannot rule out |
| G6PD deficiency | Bite cell during acute hemolytic episode | G6PD activity levels | Cannot rule out. Patients can present in adulthood |
| PK deficiency | Nucleated red cells, acanthocyte-like cells | PK activity levels | Should have presented earlier in life |
Note from the table about how helpful the peripheral smear is in differentiating causes of hemolysis! Almost every cause of hemolysis has unique findings on the smear.
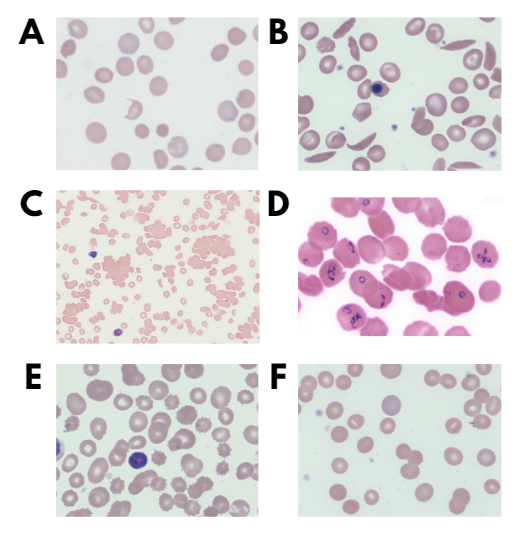

Match the smear (A-C)) with the condition:

Match the smear (D-F) with the condition:
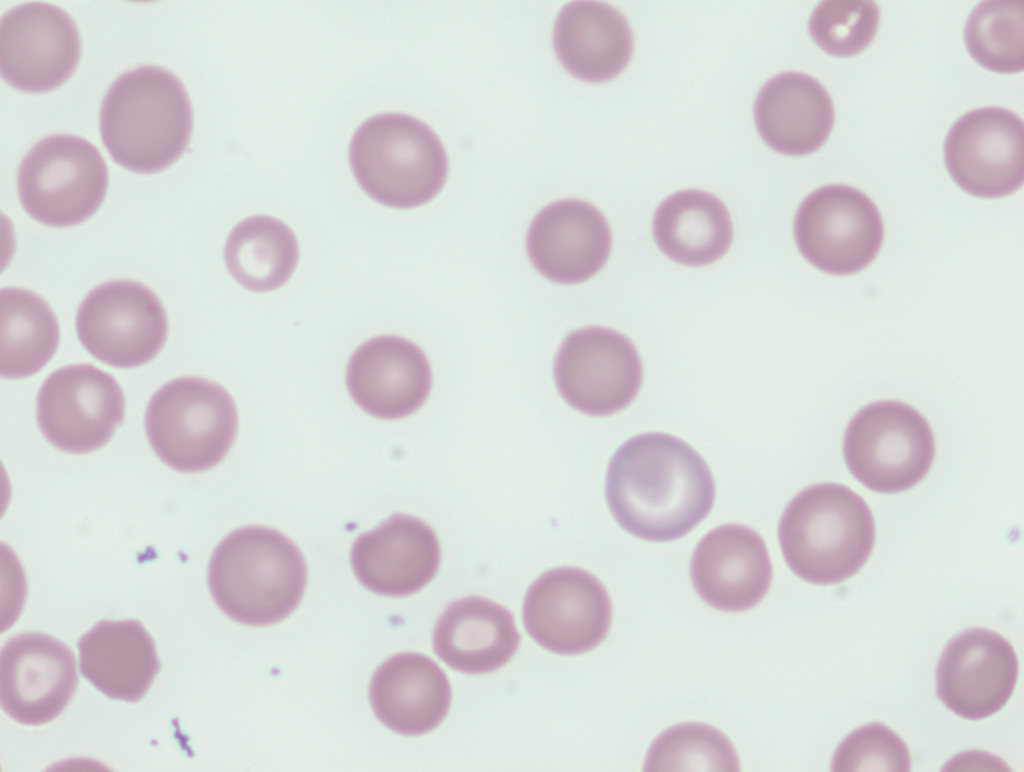
The patient’s smear performed on warmed blood is similar to the following (want to show smear with clumping at room temperature?):
Further classifying hemolytic anemias
In our diagnostic schema for hemolysis, we discussed one method to subdivide hemolysis – separating etiologies into immune and non-immune buckets, and non-immune bucket into “extrinsic”, and “intrinsic” causes. This is helpful for developing a broad differential for hemolysis. However, there are other ways to classify hemolysis; one common method is to separate these into “intravascular” and “extravascular” causes.
Extravascular hemolysis occurs within the spleen and reticuloendothelial system (RES), in a similar manner to the physiologic destruction of senescent red cells reviewed earlier in this case. However, this destruction occurs too early, often due to intrinsic defects within the red cell. For example, hereditary spherocytosis results in extravascular hemolysis – the abnormal shape and reduced flexibility of the spherocytes results in the cells getting “caught” in splenic sinusoids. As a result, they are treated the same way as aging red cells – they are phagocytosed by splenic macrophages.
On the other hand, intravascular hemolysis occurs by an entirely different mechanism. Rather than the phagocytosis described above, hemolysis occurs inside the blood vessel. For example, microangiopathic hemolytic anemias result in intravascular hemolysis. Due to the formation of microthrombi in capillaries and other small vessels, red cells are sheared apart as they try to navigate these vessels.
This distinction matters because the location of hemolysis (intravascular or extravascular) can help characterize the ways in which hemolysis will manifest.

Sort the cause of hemolysis with the predominant mechanism:
Key point: the “RBC-killer” (i.e. clot/shearing, complement/MAC, infectious organism, oxidative stress) has to be inside the blood vessel in order for the hemolysis to occur in the intravascular space.
Key point: spleen/RES-hemolysis is facilitated by mononuclear phagocytes (monocytes/macrophages) looking for structurally-abnormal or antibody-coated RBCs. This phagocytosis can result in spherocytes.
The ability of hemolytic labs to differentiate between intravascular or extravascular hemolysis is by no means perfect. At first glance, we might assume that intravascular causes of hemolysis lead to elevated leakage products including LDH, AST and plasma hemoglobin (released into the circulation by lysed cells), while extravascular causes of hemolysis lead primarily to increased indirect bilirubin (from intracellular conversion of Hb to bilirubin).
However:
- Many cause of hemolysis are associated with both intravascular or extravascular hemolysis
- Both intravascular or extravascular hemolysis may be associated with a similar biochemical profile:
- Intravascular hemolysis causes release of:
- LDH
- AST
- Hemoglobin (Hb)
- Free Hb binds haptoglobin (Hp) leading to reduced levels of Hp.
- Hb-Hp complexes are taken up by macrophages, converted to indirect bilirubin.
- Extravascular hemolysis leads to:
- Macrophage engulfment of red blood cells.
- Intracellular degradation of red cell Hb into bilirubin.
- Leakage of LDH and AST back to the circulation.
- Intravascular hemolysis causes release of:
For an in-depth explanation of Coombs testing, consider this 5-minute video.
Your patient’s results:
Coombs (DAT) positive for complement protein C3d (4+) and negative for IgG.
Cold-agglutinin screening positive. Patients may be screened for presence of cold antibodies if DAT positive for complement and/or IgG by repeating DAT at room temperature or by other methods approved by individual institutions. The result of a cold agglutinin test is typically reported as a titer, such as 1:64 or 1:512, expressed as the inverse value of the highest serum dilution at which agglutination can be detected, or the thermal amplitude. Some clinicians feel that cold agglutinin titer evaluation and thermal amplitude tests may not be necessary for many cases and can be used as supportive evidence for diagnosis.
Which of the following statement(s) is/are correct?
Our patient was found to have antibodies directed against I antigen.
The Ii antigen system is a human blood group system:
- The letter “I” was introduced in 1956 to denote the high degree of “individuality” shown by a patient whose RBCs lacked the antigen.
- Based upon a gene on chromosome 6 (IGnT gene).
- Consists of the I antigen and the i antigen.
- I and i antigens are carbohydrate structures on membrane glycoproteins and glycolipids.
- i antigen
- The predominant structure on fetal RBCs.
- Consists of a straight-chain polymer of at least two lactosamines.
- After birth, an enzyme becomes activated that can add additional lactosamine disaccharides to galactose, resulting in a branched chain structure that constitutes the big I antigen.
- I antigen
- Present on RBCs in the vast majority of the population (approximately 99 percent).
- i antigen
- The I/i antigens on RBCs exhibit a reciprocal expression profile during development: the I antigen is normally present on the cell membrane of red blood cells in all adults, while the i antigen is present in fetuses and newborns. In newborn infants the i antigen undergoes gradual conversion to reach adult levels of the I antigen within 18 months of birth.
- The function of the big I and little i antigens is unknown.
Cold agglutinins should not be confused with cryoglobulins. Cryoglobulins are immunoglobulins (with or without complement protein) that precipitate in vitro at temperatures below 37°C and redissolve on rewarming. They are not associated with hemolysis. Cryoglobulinemia syndrome should be suspected in patients presenting with:
- Arthralgia
- Purpura
- Skin ulcers
- Glomerulonephritis
- Peripheral neuropathy
A closer look at the mechanism of hemolysis in cold agglutinin disease is shown on the following slide.
There are two types of cold agglutinin disease:
- Primary (idiopathic) cold agglutinin disease (CAD):
- > 90% of patients reported to have clonal expansion of kappa-positive B cells in bone marrow and a monoclonal IgM-kappa paraprotein.
- Monoclonal cold autoantibody is often encoded by IGHV4-34 and targets I antigen.
- Secondary CAD (referred to by some as cold agglutinin syndrome):
- Arises in the setting of an underlying disorder such as
- Viral infection
- Autoimmune disorder
- Systemic lupus erythematosus (SLE)
- Rheumatoid arthritis
- Overt lymphoid malignancy
- Antibody is typically IgM and its target depends on underlying condition:
- Mycoplasma pneumonia – polyclonal IgM against I antigen
- Epstein-Barr virus – polyclonal IgM against i antigen
- Cytomegalovirus – polyclonal IgM against i antigen
- Aggressive non-Hodgkin lymphoma (NHL) – monoclonal IgM against I antigen, but light chain restriction can be lambda as well as kappa
- Arises in the setting of an underlying disorder such as
Younger patients may be more likely to have an underlying infection or autoimmune disorder, and older individuals (eg, >60 years of age) may be more likely to have a lymphoid (B-cell or plasma cell) malignancy such as aggressive non-Hodgkin lymphoma or Waldenström macroglobulinemia (WM).
Of note, infectious and inflammatory causes tend to result in a temporary surge in polyclonal antibody production, and therefore tends to produce a less severe clinical picture.
In contrast, lymphoproliferative disorders tend to result in monoclonal anti-I production, which can result in a more significant degree of anemia and symptom burden.
It is important to understand why your patient is producing anti-I antibodies. Is this a “primary” disorder (i.e. CAD), or is there another underlying process (i.e. CAS)? Because CAS can be associated with lymphoid malignancy, it is particularly important to evaluate for underlying causes. What tests would you order to distinguish primary from secondary cold agglutinin disease? (We can place here or in subsequent visit)
These tests were ordered in our patient and showed: XXXX
Remember our patient presented with acrocyanosis and livedo reticularis. Are these findings consistent with cold agglutinin disease?
