Preparing to see the patient
You have just been asked to consult on a patient in the hospital with possible hemolysis. You should be asking yourself several questions as you prepare to see the patient. For example:
- What is the evidence for hemolysis?
- How severe is the anemia?
- What is the cause of the hemolysis?
On the following slides, we will address each of these questions in turn.
Question 1 – What is the evidence for hemolysis?
Which of the following hemolytic markers are released from red cells?
The next two slides show schematics of hemolytic markers that are directly released by red cells and those that are indirectly affected by red cell release of hemoglobin.
Hemolytic markers directly released from red blood cells:
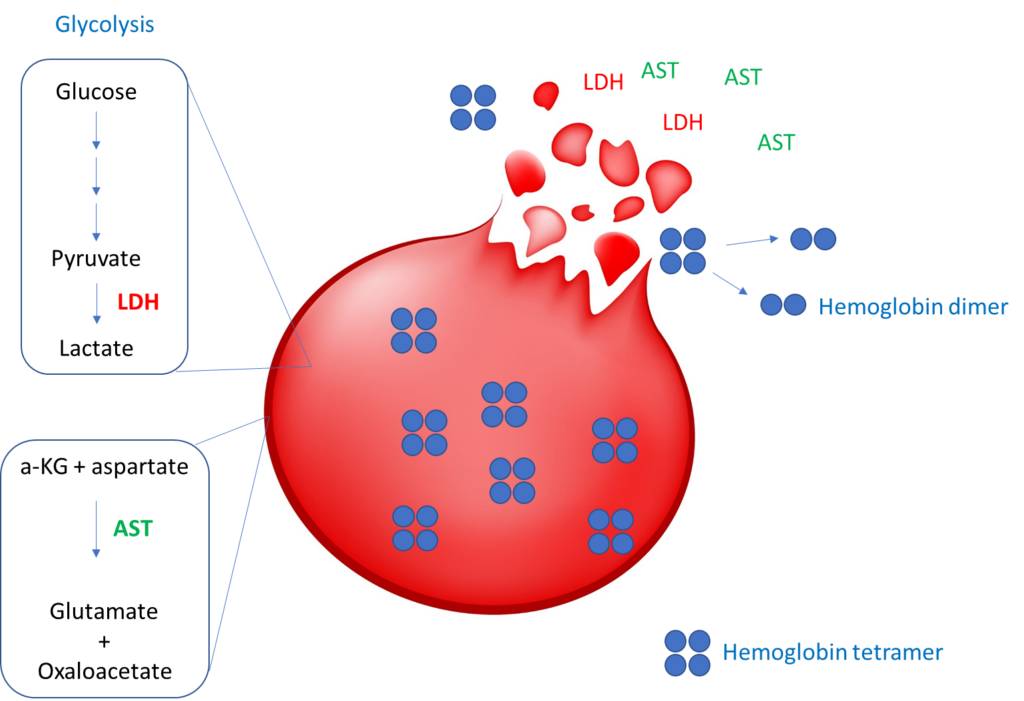
Indirect hemolytic markers (not released by red cells):
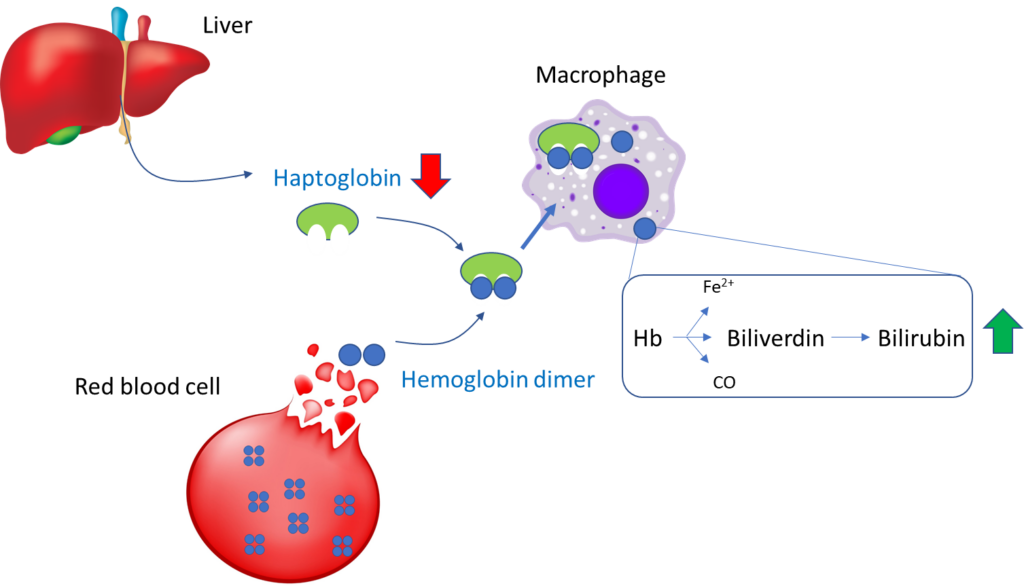
To reiterate, the following are considered serum markers of hemolysis:
- Serum LDH
- Serum bilirubin
- Serum AST
- Serum free Hb
- Serum haptoglobin
In fact, one study showed that mature erythrocytes contain 2,289 distinct gene products! Learn more here.
Note that with the exception of plasma hemoglobin, none of the hemolysis markers presently used is specific to red blood cells:
| Marker | Hemolysis | Liver disease | Rhabdomyolysis | Comments |
|---|---|---|---|---|
| Increased LDH | + | + | + | Also increased in megaloblastic anemia, HLH and LPD |
| Increased bilirubin | + | + | – | Also increased in megaloblastic anemia, HLH |
| Increased AST:ALT | + | + (especially alcoholic liver disease) | + | Also increased in megaloblastic anemia, HLH |
| Decreased haptoglobin | + | + | – | Also increased in megaloblastic anemia, HLH |
No single pattern is absolutely diagnostic of hemolysis. The markers must be interpreted in the appropriate clinical context!
Question 2 – How severe is the anemia?
There are three possible ways to express anemia. Which the is the most relevant?
You will probably agree that of the three parameters (red cell count, hemoglobin and hematocrit), the red cell count is the least useful measure of anemia. One of the reasons for this is that you could have all the red cells in the world, but if they are deficient in hemoglobin and/or smaller than normal in size, the oxygen carrying capacity of blood (which is a function of the hemoglobin concentration) may be impaired.
The same is true when thinking about hemoglobin vs. hematocrit (Hct). Hemoglobin carries oxygen. Hct is simply the fractional volume of blood that is comprised of red cells. It doesn’t tell us anything about hemoglobin levels and oxygen carrying capacity of the blood. In theory, red cells could be completely devoid of hemoglobin, yet maintain a normal Hct.
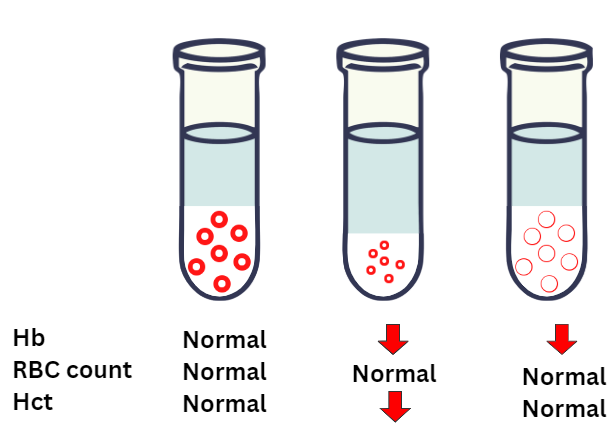
All of this is to say that we want to judge the severity of the patient’s anemia by examining her hemoglobin!
Question 3 – What is the cause of the hemolysis?
Now that you have thought about hemolytic indices, you turn your attention to etiology. Without knowing anything (beyond age and sex, and inpatient status) of the patient, and assuming she is indeed hemolyzing and the condition is acquired, which one of the following conditions are you most concerned about ruling out as soon as possible?
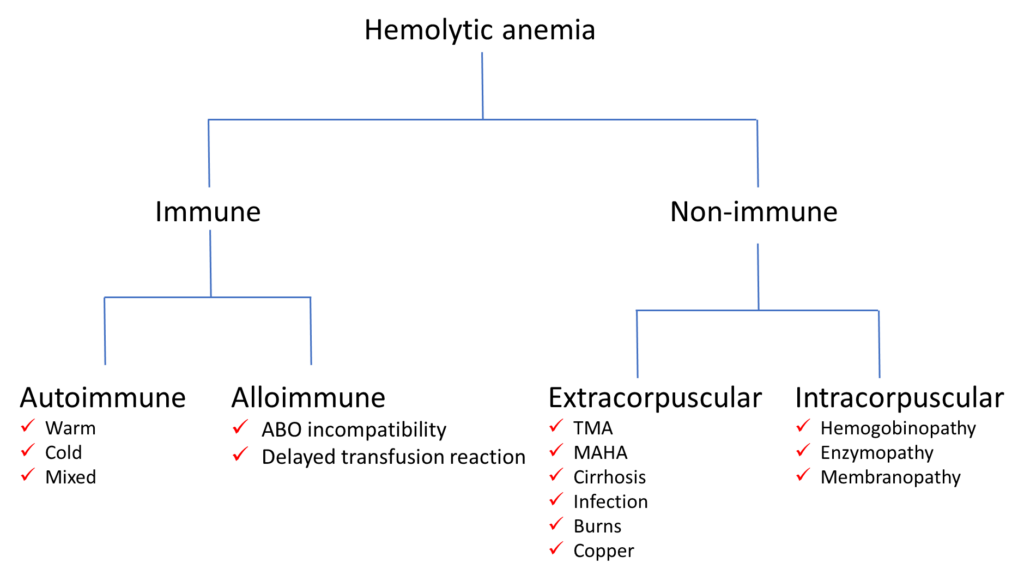
Differential diagnosis of hemolytic anemia
As you prepare to see the patient, it pays to have a broad outline of the differential diagnosis. Nothing too fancy or detailed. Something along the lines of the scheme shown below. We will discuss some of these conditions in a subsequent section when we consider the patient’s lab results.
You are now primed to see the patient! In the next series of slides, we will consider the history and physical exam.

