Labs
The following is the patient’s complete blood count (CBC) when you are asked to see her:
| WBC (109/L) | Hb (g/dL) | MCV (fL) | MCHC (g/dL) | PLT (109/L) |
|---|---|---|---|---|
| 9.4 | 7.2 | 94 | 32 | 155 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45 fL, platelets (PLT) 150-450 x 109/L
PT and aPTT results:
| PT (seconds) | INR | aPTT (seconds) |
|---|---|---|
| 19.4 | 1.8 | 40.9 |
What’s what: PT, prothrombin time; INR, international ratio; aPTT, activated partial thromboplastin time; Normal values: PT 9.4-12.5 seconds, aPTT 25-36.5 seconds
The patient has elevation in both the PT/INR and aPTT.
Let’s look at how a PT and aPTT are carried out:
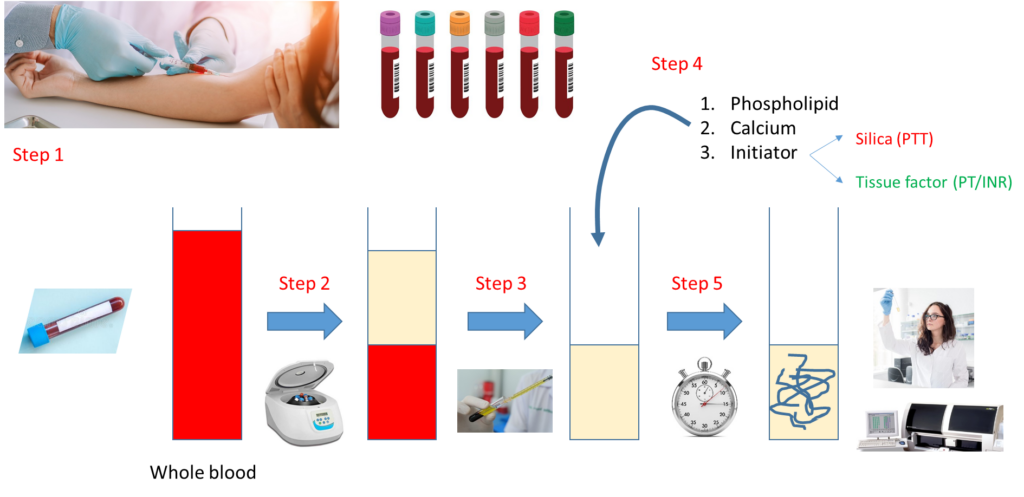
Notes:
- Step 1 – Draw whole blood from a blood vessel (typically an arm vein) into a blue top tube containing the anticoagulant, sodium citrate.
- Step 2 – Spin the liquid blood sample in a centrifuge so that red cells layer on the bottom, plasma on the top.
- Step 3 – Pipette plasma (top layer) into a clean, empty test tube.
- Step 4 – Add phospholipid, calcium and activator (tissue factor for PT, silica for aPTT).
- Step 5 – Incubate at 37 degrees C and measure time to clot formation, either with automated instrument or more rarely by vision.
Note that the major difference between the PT and aPTT is the nature of the activator added to the sample.
What are possible causes of an elevation in both the PT and aPTT?
Further work-up of this case requires some knowledge of the clotting cascade. Let’s review the basics:
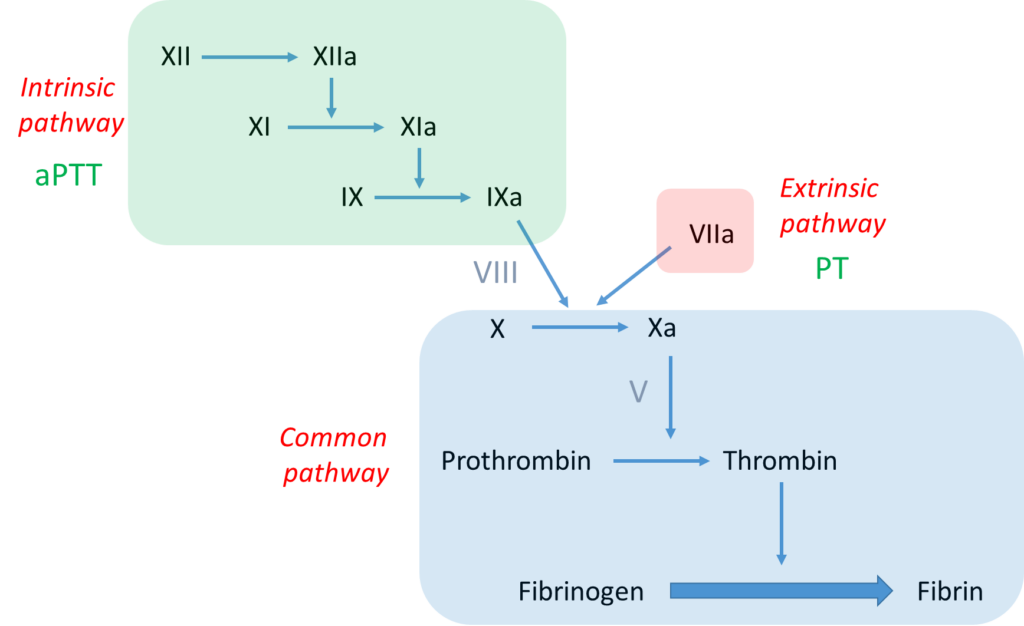
Notes:
- The bottom line is formation of an insoluble fibrin plug, which, along with platelets, stems blood loss.
- Fibrin is derived from thrombin-mediated cleavage of soluble fibrinogen (which is a structural protein).
- Thrombin (which is a type of enzyme called a serine protease) is formed when another serine protease, activated factor X (FXa), cleaves prothrombin.
- Two pathways may activate factor X:
- The intrinsic pathway, which consists of a series of linked reactions involving serine proteases FXII, FXI and FIX, and cofactor FVIII.
- The extrinsic pathway, which consists of tissue factor-mediated activation of FVII (FVIIa).
- In vivo, the clotting cascade is always initiated by tissue factor activation of FVII (extrinsic pathway) and amplified by the intrinsic pathway via cross-talk (FVIIa activates FIX) and feedback (thrombin activates FXI and FVIII) mechanisms.
- aPTT measures the integrity of the intrinsic pathway.
- PT/INR measures the integrity of the extrinsic pathway.

According to the scheme above, an elevation in both the PT and aPTT indicates a deficiency of, or inhibitor against, a clotting factor in the common pathway or multiple factors in the intrinsic and extrinsic pathways.
What would you like to test for next?
A mixing study is a reasonable next step in a patient with unexplained elevation in PT and/or aPTT. What is a mixing study?
Let’s review the basics of the mixing study:
Notes:
- Mixing study involves mixing patient plasma and pooled normal plasma in 1:1 ratio.
- The resulting mixture is then processed for clotting time (PT or aPTT) immediately (time 0) and then after 2 hours of incubation.
- A clotting factor must drop below about 30% before the PT or PTT begins to prolong.
- Therefore, even if a clotting factor is completely missing (i.e. 0% level), the 1:1 mix will restore it to 50% (equal volume of plasma containing 0% of the factor and normal pooled plasma containing 100% of the factor), more than enough to restore a normal PT or aPTT (the example above shows 10% activity of imaginary factor Y in patient plasma, with 1:1 mix resulting in 60% activity).
- However, if there is an inhibitor or antibody to a clotting factor, the inhibitor will carry over from patient to normal plasma in the mix and interfere with factor activity, leading to continued prolongation of the PT or aPTT.
Mixing study (50:50 mix) results
(baseline aPTT 40.9, PT 19.4)

What is the result of the above mixing study?
To summarize at this point, we have an elevated PT and aPTT in a patient with nephrotic-range proteinuria and renal dysfunction who bled following a kidney biopsy. The mixing study points to a factor deficiency.
The first factors that were assayed were those in the common pathway, namely factor V (FV) and factor X (FX):
| Factor V (%) | Factor X (%) |
|---|---|
| 126 | 9 |
Thus, the patient has factor X deficiency.
Which of the following is/are possible causes of the factor X (FX) deficiency?
What are possible causes of acquired factor X (FX) deficiency?
To summarize, acquired FX deficiency may be caused by:
- Vitamin K deficiency
- Liver disease
- Amyloidosis
In this case, there was no previous (baseline) PT and aPTT for comparison. There was no past history of bleeding episode, nor was there a family history of bleeding. The patient was not taking a vitamin K antagonist, she had not received antibiotics (which may cause vitamin K deficiency) and she was eating a regular diet. She received vitamin K, but there was no response in her PT and aPTT. She had no history of liver disease, and her liver function tests were normal. CT abdomen showed a normal appearing liver.
By process of elimination, the suspicion for AL amyloidosis is high. Let’s consider that diagnosis:
- AL amyloidosis is a systemic disease resulting from an underlying plasma cell dyscrasia that causes deposition of misfolded amyloid light chain (AL) proteins in various organs and tissues leading to progressive organ damage:
- Heart:
- Congestive heart failure
- Cardiomyopathy
- Increased mean ventricular wall thickness
- Kidney – 24-hour urine protein ≥ 0.5 g/day, mainly albumin
- Nervous system:
- Symmetric lower extremity sensorimotor peripheral neuropathy
- Autonomic neuropathy with orthostatic hypotension
- Soft tissues:
- Macroglossia
- Arthropathy
- Skin lesions
- Carpal tunnel syndrome
- Hematological – deficiency of factor X and other clotting factors via binding to and sequestration by amyloid fibrils
- Heart:
- Most patients are diagnosed between ages 50 and 70 years.
- Caused by neoplastic plasma cell or B-cell clone produces toxic precursor protein whose aggregates deposit as interstitial fibrils of amyloid.
- Diagnosis:
- Serum protein electrophoresis (SPEP) and immunofixation (IFE) – patients with AL amyloidosis typically have little intact monoclonal Ig (some have light chains only).
- Serum free Ig light chain (FLC) assay – positive result in AL amyloidosis shows elevated level of either kappa or lambda together with altered ratio of free kappa to free lambda light chain.
- Urine protein electrophoresis (UPEP)
- Biopsy of involved organ or surrogate site:
- Abdominal subcutaneous fat, bone marrow, and minor salivary gland are the surrogate sites most often used for amyloid detection.
- Congo red staining is used to verify amyloid deposition.
The patient did not have any cardiac symptoms, or symptoms associated with autonomic or sensorimotor neuropathy. She did not have macroglossia or skin lesions. The results of her serum protein electrophoresis (SPEP) and immunofixation (IFE) were the following:

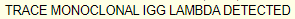
Her renal biopsy results showed:
- Amyloidosis, consistent with AL (lambda) type:
- Massive infiltration/deposition by PAS negative material that is Congo red positive with apple green birefringence.
- Amyloid stained for lambda exclusively.
- Mild to moderate interstitial fibrosis and tubular atrophy.
In summary, the patient has acquired factor X (FX) deficiency secondary to AL amyloidosis.

