Workings backward from an elevated MCV
The patient has an MCV of 121 fL.
Macrocytosis is defined as a mean cell volume (MCV) of ≥ 100 fL. Which questions in the history are relevant (more than one answer may apply)?
This patient has no history of liver disease. He is not a drinker. There is no evidence of active bleeding. Let’s ask some more questions. Which of the following are relevant?
Let’s now consider the history:
A 56-year-old otherwise healthy gentleman presents to the Emergency Room with acute right lower extremity leg pain. He was in his usual state of health until the previous week when he woke up with dull achy pain in his right calf and behind his right knee. The pain progressed throughout the day and caused him to start limping. The next day, his right leg became swollen. The leg felt heavy and he was having difficulty ambulating to the bus for his ride home. He took 2 baby aspirin this morning at home hoping this might help the symptoms. However, the pain and swelling progressed and so he decided to come to the emergency department. Routine blood work included a complete blood count (CBC), which revealed the presence of macrocytosis. He has no past medical history and has not had any surgery. He is a nonsmoker, and drinks alcohol socially. His mother had a history of deep venous thrombosis. He is not taking any medications and does not have any known allergies to drugs.
The patient’s physical exam shows a swollen right leg from the ankle to the top of the thigh, similar to the image below (showing right calf).
Which of the following conditions is/are associated with macrocytosis?
The following is a more complete differential diagnosis for macrocytosis:
- Increased reticulocytes
- Vitamin B12 deficiency
- Folate deficiency
- Medications
- Alcohol
- Liver disease
- Hypothyroidism
- Aplastic anemia
- Plasma cell dyscrasias
- Myelodysplastic syndrome
- Down syndrome
Is it possible that he has macrocytosis without anemia?
This is what you have been waiting for… the patient’s complete blood count (CBC)!
| WBC (109/L) | Hb (g/dL) | MCV (fL) | RDW-SD (fL) | PLT (109/L) |
|---|---|---|---|---|
| 5.7 | 9.5 | 120 | 67 | 154 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45 fL, platelets (PLT) 150-450 x 109/L
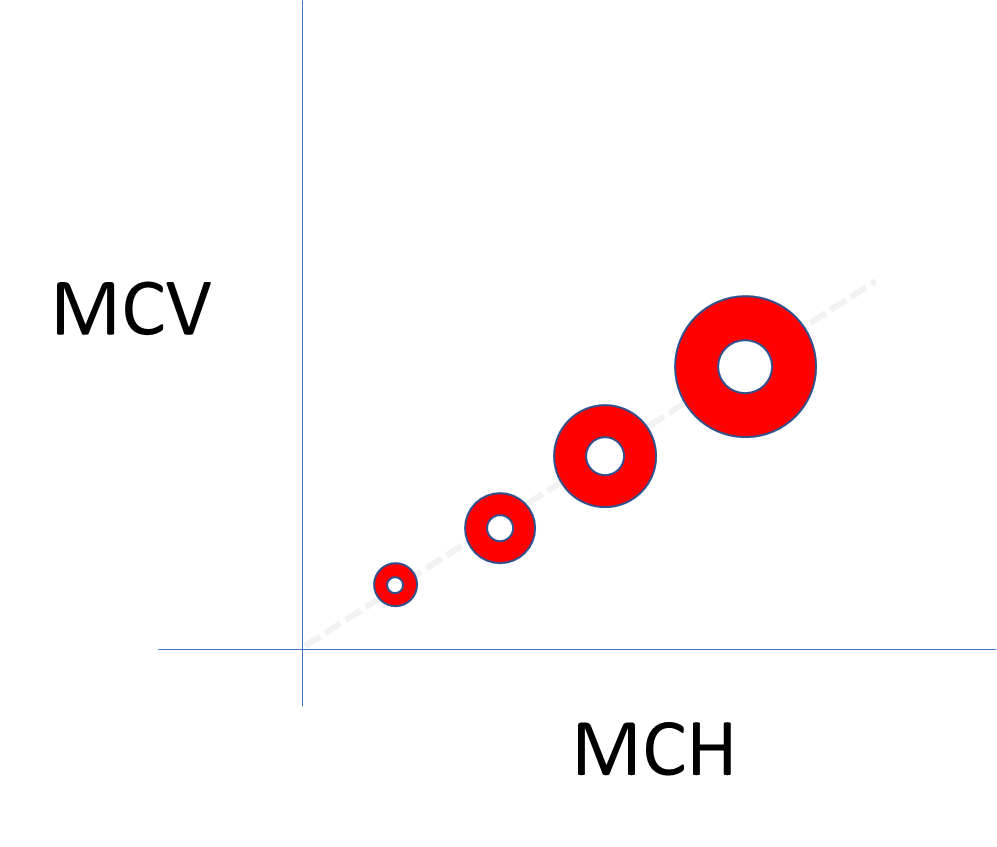
Note that the mean corpuscular hemoglobin (MCH) is not provided in the CBC above. Why? Because it really does not add anything.
Relationship between MCH and MCV (assuming a constant MCHC):
The white cell count (WBC) was normal. Does that mean there is no need for a white cell differential?
The following three slides show examples of a normal white cell count with an abnormal differential. To properly interpret these data, we need a ‘cheat sheet’ of threshold values, which is included with each example. The white cell differential is expressed in two ways: as a percentage of the total white cell count (e.g., neutrophils constitute 60% of 5 x 109/L white cells) or as an absolute number (e.g., the absolute number of neutrophils is 60% x 5 x 109/L white cells = 3.0 109/L). When considering the 5 normal white cell subsets, focus on the absolute counts. Why? Because an elevation (cytosis) or reduction (cytopenia) in counts is defined by deviation in absolute counts!

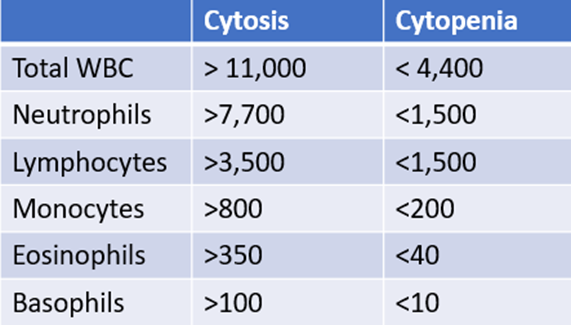
Example 1
Example 2
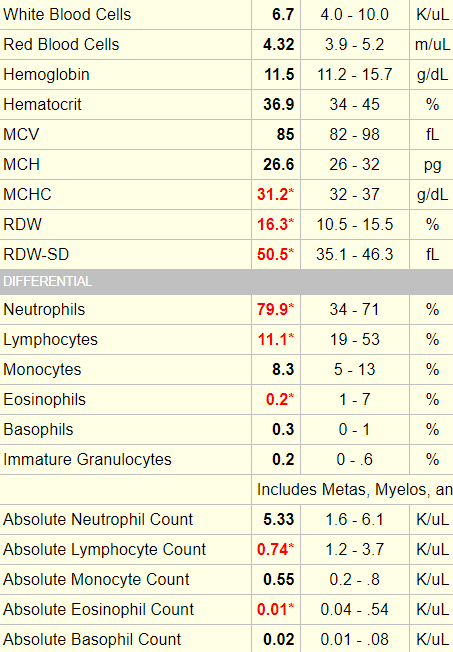
Example 3
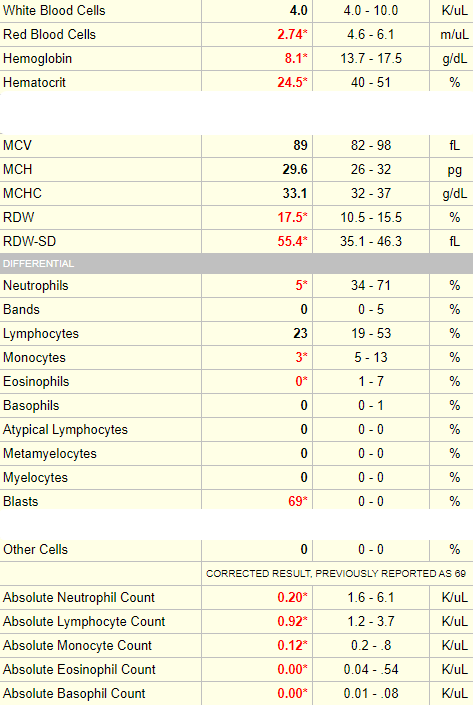
Note that in this case, blasts are reported only as a percentage of the total white cell count. While it is advisable to consider absolute counts for mature cells (neutrophils, lymphocytes, monocytes, eosinophils and basophils), it is common practice to refer to the percentage of immature white cells (including bands, metamyelocytes and myelocytes, promyelocytes and blasts) because if they are present, they are usually at low numbers (though in this case, the blast count is VERY high and could easily be converted into a meaningful absolute count).
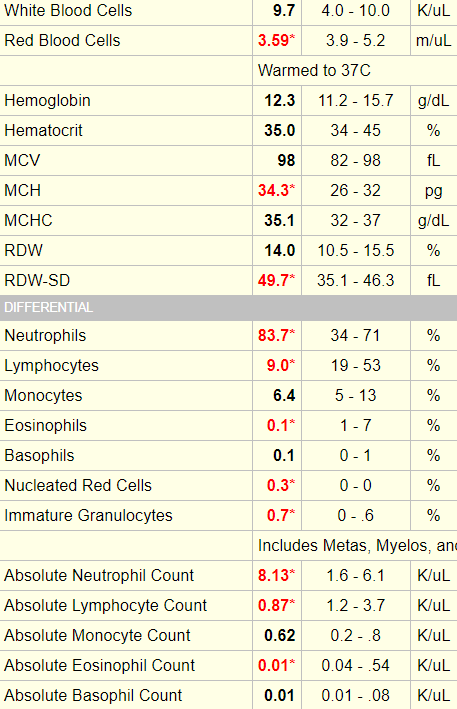
Let’s return to our patient. Here is his white cell differential:
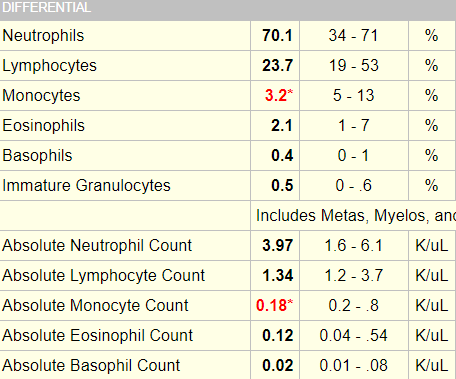
In this case, the patient’s differential is normal with the exception of a slight non-specific reduction in monocyte counts.

What is the next test you would like to order (not including the peripheral smear)?
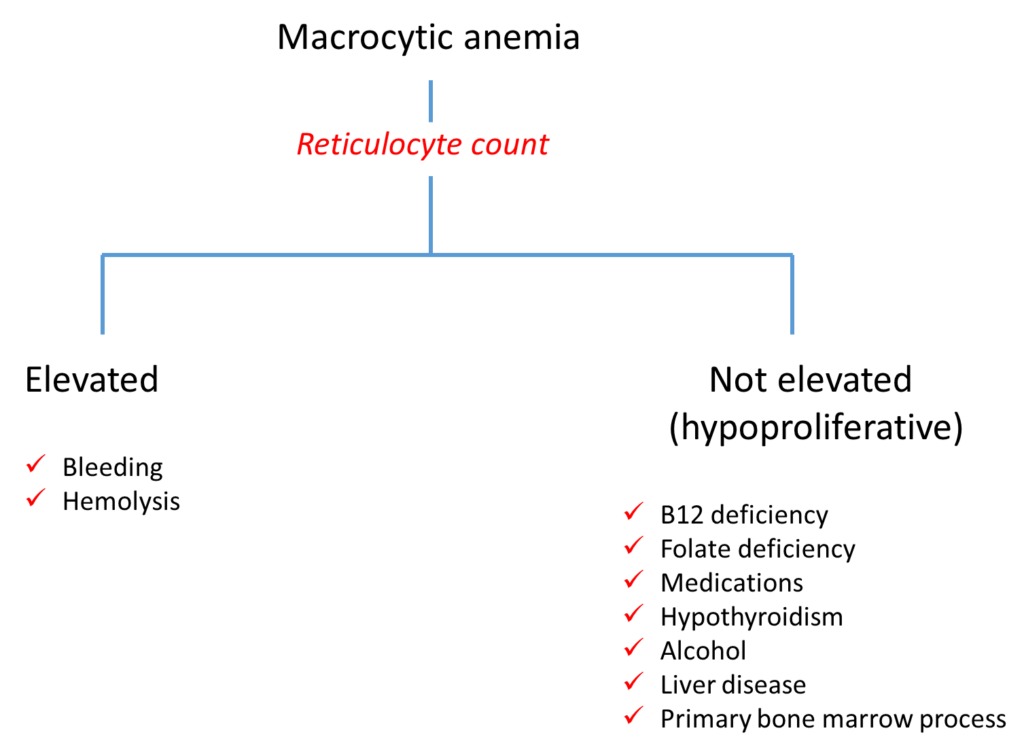
Click for AnswerThat’s right, the reticulocyte count is the first branch point in the diagnostic algorithm for macrocytic anemia!
Here is the patient’s reticulocyte count, expressed as a percentage of red cells and as an absolute count:
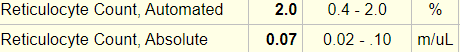
As we discussed earlier for the white cell count, the absolute number of reticulocytes provides more useful information than the percentage (which requires a fudge factor to account for the degree of anemia).
As a general rule of thumb, a reticulocyte count > 120 x 109/L is an appropriate bone marrow response to anemia.
In this case, the absolute reticulocyte is reported as 0.07 m/uL, which is 0.07 x 1012/L or 70 x 109/L. Thus, this patient has an inappropriate reticulocyte response. The anemia is defined as hypoproliferative.
Does the low reticulocyte count rule out an acute bleed or hemolysis?
During your initial lab work, you find that the patient’s serum haptoglobin is undetectable. What are some possible explanations for this?
There is no evidence of liver disease, and there is no history of hematoma, dark urine or jaundice. You find the additional lab results:
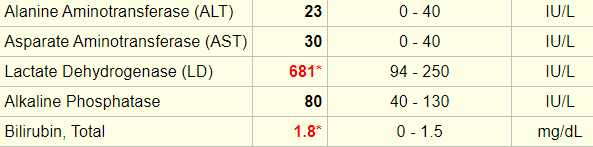
Let’s explore the diagnostic algorithm for hemolysis (we’ll get to the peripheral smear shortly!).
Here is one way to classify hemolytic anemia:
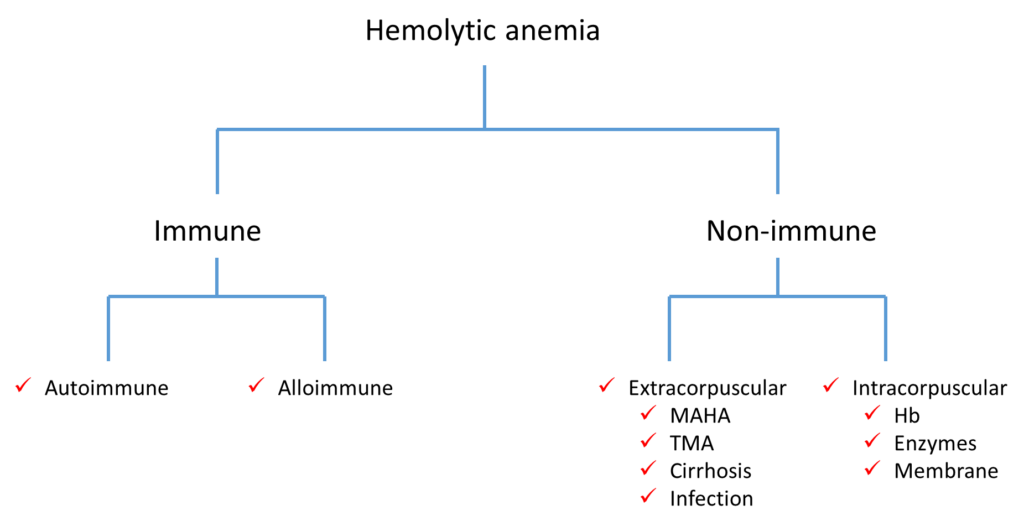
If you replace the term hemolytic anemia at the top of the algorithm with positive hemolytic markers, what other types of conditions would you add to this scheme?
Now we can take a look at the peripheral smear:
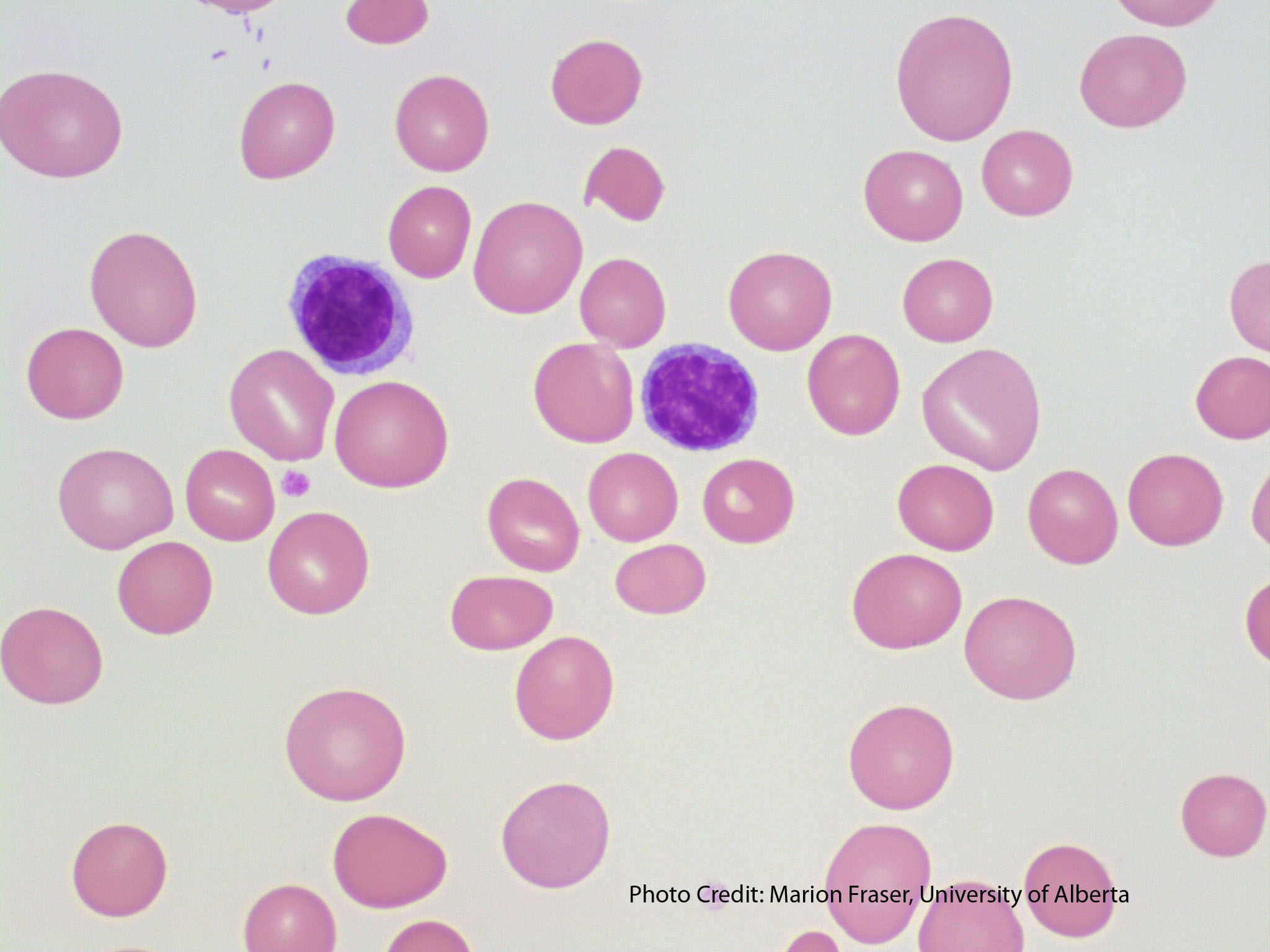
The peripheral smear of this patient was similar to the one shown above. There were lots of macro-ovalocytes and occasional fragmented red cells.
The peripheral smear also showed occasional hypersegmented neutrophils, similar to the image below:
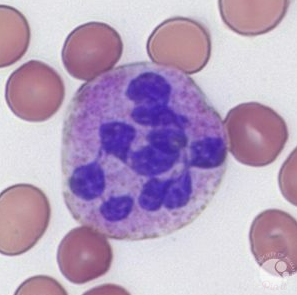
Hypersegmented neutrophils:
- Suggested by > 5% of neutrophils with ≥ 5 lobes or 1% with 6 lobes.
- Supports diagnosis of megaloblastic anemia (especially vitamin B12 and folate deficiency).
- Typically appear before macrocytosis and anemia.
Let’s return to the differential diagnosis of macrocytosis again:
- Increased reticulocytes
- Vitamin B12 deficiency
- Folate deficiency
- Medications
- Alcohol
- Liver disease
- Hypothyroidism
- Aplastic anemia
- Plasma cell dyscrasia
- Myelodysplastic syndrome
The table below presents a differential diagnosis of macrocytosis with findings in our patient that support or do not support one or another diagnosis:
| Disorder | For | Against | Possible additional tests |
|---|---|---|---|
| Increased reticulocytes | N/A | Inappropriate retic response | N/A |
| Vitamin B12 deficiency | Smear findings, hemolytic markers | N/A | See below |
| Folate deficiency | Smear findings, hemolytic markers | Very rare now that food is fortified | Folate levels |
| Medications | N/A | No history of medication use; hypersegmented neutrophils would be unusual. | N/A |
| Alcohol | N/A | Negative history, anemia not common (typically isolated macrocytosis); hypersegmented neutrophils would be unusual. | AST:ALT |
| Liver disease | N/A | No history or symptoms; no thrombocytopenia; hypersegmented neutrophils would be unusual. | Liver function tests |
| Hypothyroidism | N/A | No history or symptoms; hypersegmented neutrophils would be unusual. | TSH |
| Aplastic anemia | N/A | Normal white cell and platelet count; hypersegmented neutrophils would be unusual. | Bone marrow biopsy |
| Plasma cell dyscrasia | N/A | N/A | SPEP, bone marrow biopsy |
| Myelodysplastic syndrome | N/A | N/A | Bone marrow biopsy |
Here are the results of the patient’s serum vitamin B12 and folate:
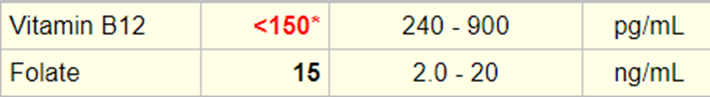
Definition of vitamin B12 deficiency:
- British Committee for Standards in Haematology (BCSH) guideline suggests using cutoff of < 148 pmol/L (200 pg/mL) or cutoff derived from local reference range in patient with strong clinical suspicion of vitamin B12 deficiency.
- British Columbia (Canada) Medical Association suggests probability of vitamin B12 deficiency high with cutoff < 75 pmol/L (102 pg/mL), moderate with cutoff 75-150 pmol/L (102-203 pg/mL), low with cutoff > 150 pmol/L (203 pg/mL).
Serum vitamin B12 (cobalamin) level:
- Preferred initial test for detecting vitamin B12 deficiency.
- Assay measures both active form of cobalamin (holotranscobalamin) and inactive form (holohaptocorrin).
- Assay for measuring serum vitamin B12 is not standardized and there is no consensus cutoff to define deficiency.
We will come back to this figure in the next section (Pivoting from blood to stomach), but the important point to make here is that vitamin B12 is carried in the blood by two binding proteins (transcobalamin [TC] and haptocorrin [HC]), and only one of these (TC) carries bioavailable B12. The vitamin B12 test assays both TC- and HC-bound vitamin B12. Thus, high HC levels may mask B12 deficiency.
Are you familiar with functional assays for vitamin B12 deficiency? There are two of them.
The reason these two metabolites are increased in vitamin B12 deficiency is depicted in the following scheme which shows the two B12-dependent enzymatic reactions in the body:
- Methionine synthase reaction in the cytoplasm – conversion of homocysteine to methionine allows recycling of 5-methyl-tetrahydrolfolate (THF) to N5,10 methylene-THF which is necessary for generation of thymidylic acid, that is then used for DNA synthesis. Homocysteine levels build up when vitamin B12 levels are low.
- Methylmalonyl CoA mutase reaction in the mitochondria – conversion of methylmalonyl CoA (generated from propionic acid by bacteria-derived propionic acid) to succinyl CoA, a precursor for heme and fatty acid synthesis. Methylmalonic acid levels build up when vitamin B12 levels are low.