Imaging and Labs
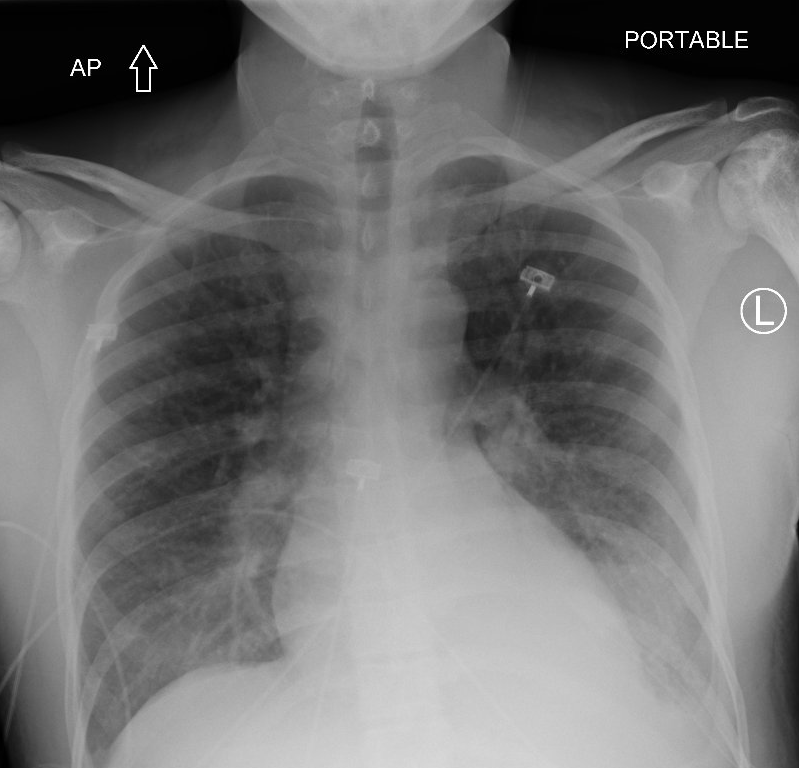
The next slide shows the patient’s chest X-ray (and its interpretation by the radiologist) at the time you see her in the emergency department.

The following is a partial complete blood count (CBC) on the day of admission and her baseline CBC for comparison:
| Day | WBC (109/L) | Hb (g/dL) | MCV (fL) | PLT (109/L) |
|---|---|---|---|---|
| Day of transfer | 15.4 | 6.9 | 75 | 92 |
| 6 months prior | 8.1 | 12.3 | 73 | 155 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45%, platelets (PLT) 150-450 x 109/L
We are dealing with a patient with sickle cell disease:
- Why is she microcytic at baseline?
- Why is her baseline Hb so high?
- Why is her platelet count low?
Food for thought. We will come back to these questions.
The white cell differential:
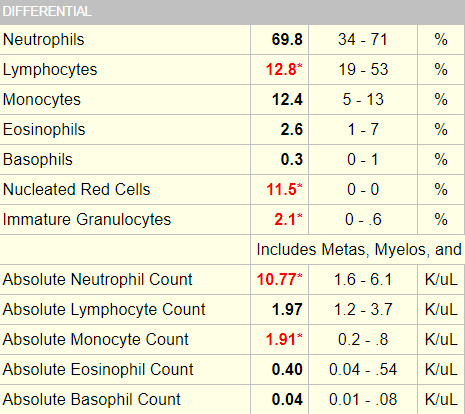



Also note the nucleated red cells, which are characteristic of patients with sickle cell disease.
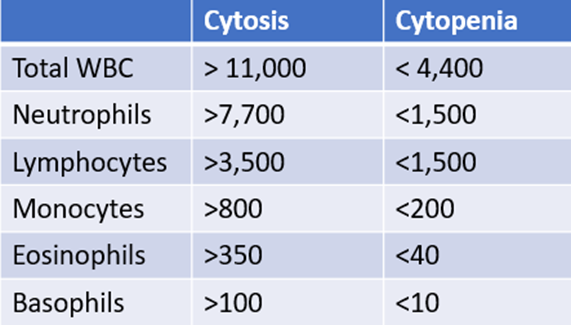
How do you know which white cell values are high or low? Because you carry around the following cheat sheet on your iPhone!

Reticulocyte count on day of admission:
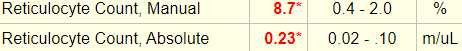

Ignore the percentage! Focus on the absolute reticulocyte count. Absolute reticulocyte count = 0.23 x 1012/L = 230 x 109/L
So, the patient’s reticulocyte count is appropriate. What diagnosis would you entertain if the absolute reticulocyte count was 0.01 x 1012/L?
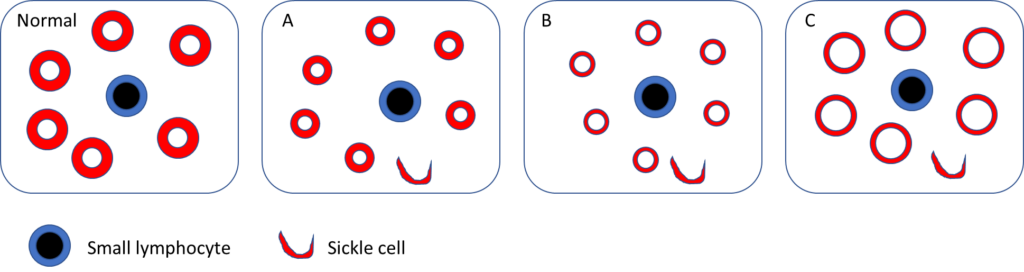
Which peripheral smear is most likely to resemble the patient’s?
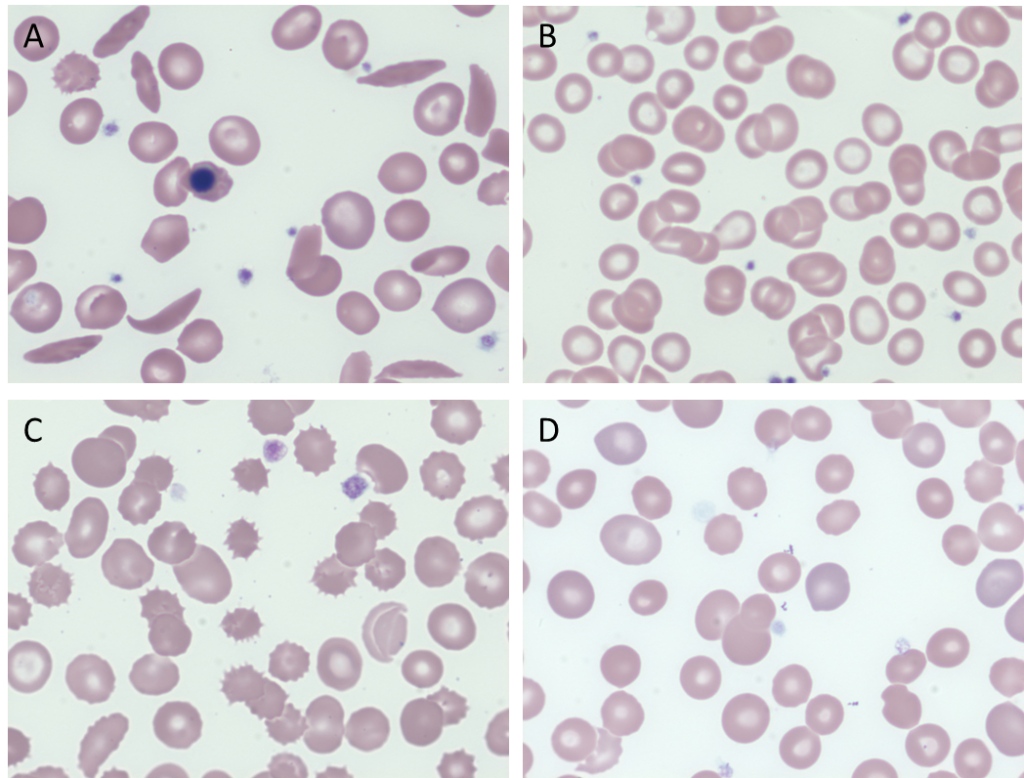

True of false: the number of sickle cells on the peripheral smear of a patient with sickle cell disease is demonstrably greater during a vaso-occlusive (pain) crisis or acute chest syndrome.
What are possible explanations for the low mean cell volume (MCV)?
How would the mean corpuscular hemoglobin concentration (MCHC) help distinguish between these various causes of microcytosis in a patient with sickle cell disease?
Let’s look at the patient’s mean corpuscular hemoglobin concentration (MCHC):
| Day | Hb (g/dL) | MCV (fL) | MCHC (g/dL) |
|---|---|---|---|
| Day of transfer | 6.9 | 75 | 35.8 |
| 6 months prior | 12.3 | 73 | 36.4 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45%, platelets (PLT) 150-450 x 109/L
These results are most consistent with:
Which blood smear schematic is most consistent with the patient’s?

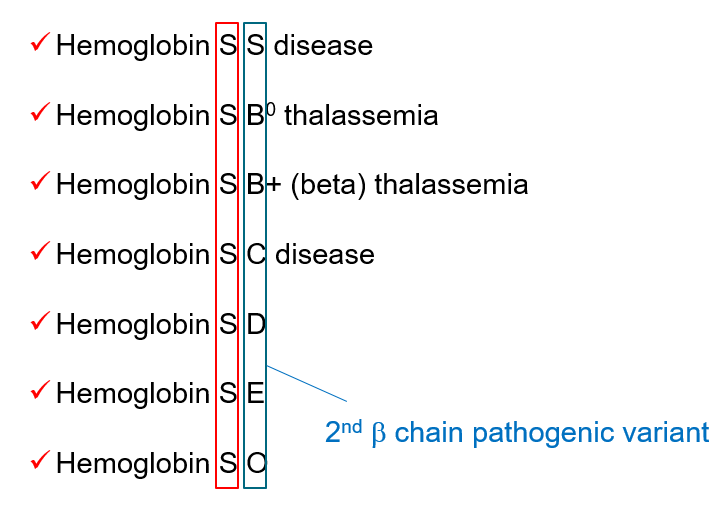
Let’s pause for a moment and consider genotype-phenotype correlations in sickle cell disease as they pertain to HbSS and HBSC.
Definitions of sickle cell disease and sickle cell anemia
- Sickle cell disease (SCD) refers to a group of disorders characterized by the presence of at least one hemoglobin S allele (HbS; p.Glu6Val in HBB) and a second HBB pathogenic variant resulting in abnormal hemoglobin polymerization. SCD includes:
- Homozygous for HbS (HbSS)
- Sickle beta0 thalassemia (compound heterozygote with one sickle gene and one beta0 thalassemia gene)
- Hemoglobin SC disease (HbSC) (compound heterozygous)
- Sickle beta+ thalassemia (compound heterozygous)
- Rarer forms, including:
- SD-Punjab (also known as HbSD)
- SO-Arab
- SC-Harlem
- S-delta-beta-thalassemia
- SE
- S-Lepore

- Sickle cell anemia is a subset of sickle cell disease that refers to individuals who are either of the following:
- Homozygous for HbS (HbSS)
- Sickle beta0 thalassemia (compound heterozygote with one sickle gene and one beta0 thalassemia gene)


Hemoglobin SC
- A genotype of sickle cell disease (SCD).
- Caused by co-inheritance of the hemoglobin S (HbS) and hemoglobin C (HbC) beta globin gene (HBB) mutations.
- Accounts for 30% of sickle cell disease (SCD) in the United States.
- Survival of patients with HbSC is superior compared to SCA.
- Pathophysiological processes include:
- Altered K–Cl cotransporter contributing to RBC dehydration, which:
- Increases the intracellular hemoglobin concentration
- Makes the cell more dense than HbAA-containing red cells
- Enhances the pathogenic properties of HbS
- Polymerization of HbS subunits
- Crystallization of HbC subunits
- Altered K–Cl cotransporter contributing to RBC dehydration, which:
- Improved outcome with increased levels of HbF or co-inheritance of alpha thalassemia gene deletions.
- Compared with patients with HbSS, those with HbSC have:
- Higher mean Hb
- >70% of people with HbSC have anemia
- Only 10% have Hb lower than 10 g/dL
- Lower mean corpuscular volume (MCV) (inheritance of alpha thalassemia trait further reduces MCV)
- Increased MCHC
- In some but not all patients
- Average MCHC in HbC disease, HbSC disease, HbAC trait, and HbA cells 38, 37, 34, and 33 g/dl, respectively
- Higher mean Hb
- Lower absolute reticulocyte count
- 2-fold greater red cell life span
- Increased blood viscosity
- Increased incidence of
- Proliferative sickle retinopathy
- Acute splenic sequestration crisis (chronic splenomegaly is associated with thrombocytopenia in 35% of children and 50% of adults with HbSC and may cause recurrent abdominal pain)
- Peripheral blood smear may show:
- Target cells
- Irregularly contracted cells
- Boat shaped cells (but few classic sickle cells)
- Microcytic and hyperchromic red cells
- Microspherocytes
- Red cells with crystalline inclusions
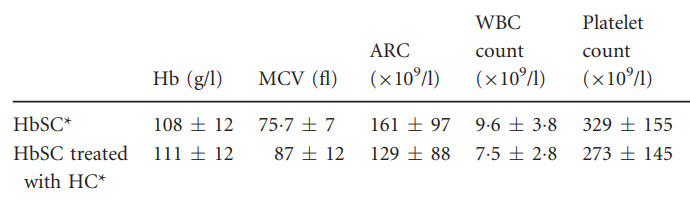
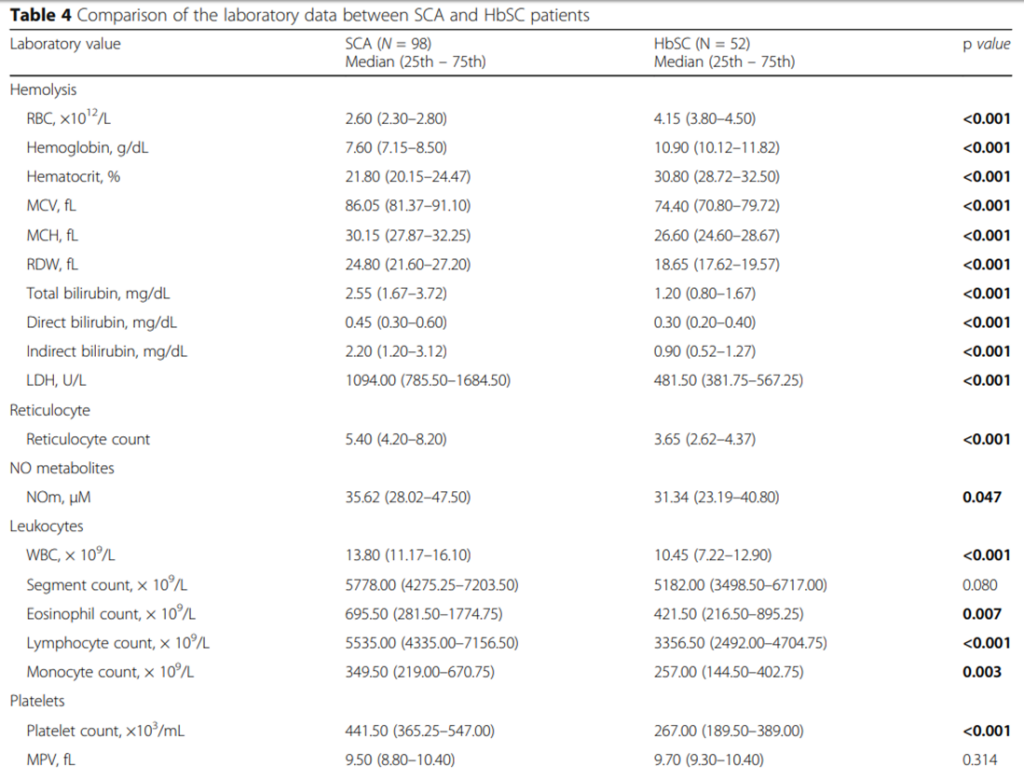
The following are complete blood count data and hemolysis markers comparing HbSC and HbSS:


OK, we have a complete blood count, white cell differential and reticulocyte count in a patient with known sickle cell disease (with complete blood count data pointing towards HbSC) and suspected acute chest syndrome. What other tests do we want to order? Let’s look at guideline recommendations from the British Committee for Standards in Haematology (next slide).
The British Committee for Standards in Haematology guideline on the management of acute chest syndrome (ACS) in sickle cell disease (SCD) recommends the following tests in all patients with suspected acute chest syndrome:
- Chest X-ray
- Complete blood count
- Liver and kidney function tests
- Blood group and screen (or crossmatch)
- Arterial blood gas on room air in adults if oxygen saturation <95% on room air
- Serology for atypical respiratory organisms including:
- M. pneumoniae
- Chlamydophila pneumoniae
- Legionella
- Blood cultures
- Serology for atypical respiratory organisms
- Urine for Pneumococcal and Legionella antigen
- Sputum for bacterial culture
Additional recommendations, if clinically indicated:
- Sputum and nasopharyngeal aspirate for viral testing including:
- Influenza A (and H1N1 subtype)
- Influenza B
- Metapneumovirus
- Adenovirus
- Parainfluenza
- RSV
- B19 parvovirus serology
Computerized tomography (CT) of the chest and V/Q scanning are not helpful in the acute setting. A CT pulmonary angiogram (CTPA) is recommended if there is a high clinical suspicion of pulmonary embolism.
Before moving to the other labs, let’s return to the observation that this patient presented with thrombocytopenia.
Patients with sickle cell disease and vaso-occlusive crisis (VOC) or acute chest syndrome (ACS) may demonstrate a decline in platelet count.
In one study, thrombocytopenia was found to be more common than thrombocytosis in severe sickle cell crisis.
Another study showed that a platelet count drop more than 10% from steady state to admission in a patient with VOC was an independent predictor for developing ACS. Patients who developed ACS tended to have a decline in platelet count from steady state at the time of presentation whereas those who did not tended to have an increase in platelet count at presentation. The authors proposed the following mechanisms:
- Consumption of platelets in a disseminated intravascular coagulation (DIC)-like picture
- Sepsis or to a viral syndrome
- Acute splenic sequestration or hypersplenism
In another study, a drop in platelet count from baseline was found to correlate with time to death in patients with sickle cell anemia who died of multi-organ failure.
In summary, the patient’s thrombocytopenia is consistent with her acute presentation.
Liver function tests, urea and creatinine were normal in our patient.
The following are the hemolysis labs:
| Baseline (1 month earlier) | Day of transfer | |
|---|---|---|
| LDH (IU/L) | 203 | 1461 |
| AST (IU/L) | 14 | 73 |
| ALT (IU/L) | 8 | 12 |
| Haptoglobin (mg/dL) | 44 | <10 |
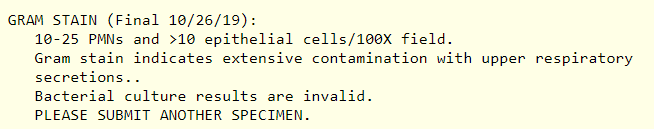
Gram stain sputum:

Arterial blood gas was not performed.
Does the patient have acute chest syndrome?
To answer this question, we need to consider the definition of the condition. According to the NHLBI, a person with acute chest syndrome (ACS) typically has both:
- Sudden onset of signs and symptoms of lower respiratory tract disease, for example some combination of:
- Cough
- Shortness of breath
- Retractions
- Rales
- A new pulmonary infiltrate on chest radiograph
The following are characteristic features of ACS:
- Children usually have fever and upper or middle lobe involvement, whereas adults are often afebrile and present with multilobe disease.
- There are no distinctive laboratory features of ACS, although the hemoglobin concentration often declines sharply below the patient’s baseline value.
- What would be considered pneumonia in a person without sickle cell disease (SCD) usually fulfills the criteria for ACS.
According to the British Committee for Standards in Haematology (BCSH), ACS is defined as an acute illness characterized by fever and/or respiratory symptoms, accompanied by a new pulmonary infiltrate on chest X-ray.
Based on this information, does the patient have acute chest syndrome?
Nearly half of patients with acute chest syndrome (ACS) present initially with a painful vaso-occlusive crisis and then develop this complication while in hospital. That was not the case with our patient. She presented with de novo ACS.
In summary, you have made a diagnosis of acute chest syndrome in this patient with sickle cell disease (who does indeed have HbSC). Now it’s time to consider management.


