What is Neutropenia?
Neutropenia is a reduction in the absolute neutrophil count (ANC), typically below 1.5 × 10⁹/L. It is not a single entity but a clinical finding that requires classification to guide evaluation and management. The hematologist’s role is to consider severity, duration, cause, and dynamics when interpreting neutropenia and deciding next steps.
- Definition: ANC < 1.5 × 10⁹/L.
- Not a diagnosis in itself, but a sign that requires classification and context.
Definitions
- Neutrophils are also called polymorphonuclear neutrophils (PMN), polymorphonuclear leukocytes, or granulocytes.
- Neutropenia:
- An abnormally low number of circulating neutrophils in the peripheral blood, with an absolute neutrophil count (ANC) ≤ 1.5 × 109/L in adults and children aged > 12 months, ANC < 2 × 109/L in infants aged 2-12 months, and < 2.5 × 109/L in neonates and infants aged < 2 months.
- Mean neutrophil counts may vary by ethnicity. Patients of African or Middle Eastern descent may have lower neutrophil counts at baseline, with 4.5% of persons of African descent reported to have ANC < 1.5 × 109/L.
- Agranulocytosis is defined as ANC ≤ 0.2 × 109/L, which carries a risk of severe infections with susceptibility to opportunistic organisms.
Classifications of Neutropenia
- By Severity – severity determines risk of infection and urgency of intervention:
- Mild: ANC 1.0–1.5 × 10⁹/L
- Moderate: ANC 0.5–1.0 × 10⁹/L
- Severe: ANC < 0.5 × 10⁹/L
- Agranulocytosis: ANC < 0.2 × 10⁹/L → very high risk of life-threatening infections
- By Duration – the timeline of neutropenia provides key diagnostic clues:
- Acute/transient: often related to infection, drug exposure, or transient marrow suppression.
- Chronic (≥3 months): may reflect autoimmune neutropenia, congenital causes, marrow failure syndromes, or chronic infections.
- By Etiology:
- Acquired: the majority; due to drugs, infections, autoimmune processes, marrow infiltration, nutritional deficiencies.
- Congenital: rare syndromes such as severe congenital neutropenia, cyclic neutropenia, Shwachman-Diamond syndrome, GATA2 deficiency.
- By Dynamics:
- Permanent: ANC persistently low, as in congenital marrow failure or aplastic anemia.
- Intermittent: fluctuating counts, as in cyclic neutropenia or autoimmune neutropenia with waxing and waning levels.
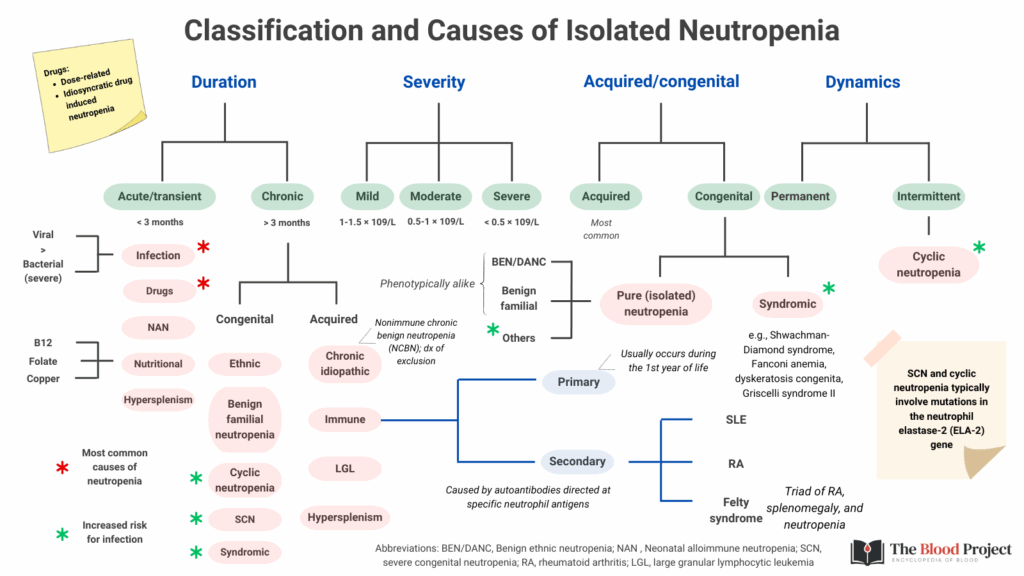
For larger image, click here.
Epidemiology
- The reported prevalence of a neutrophil count < 1.5 × 109/L:1
- 4.5% of Black persons
- 0.79% of White persons
- 0.38% of Mexican American persons
- Higher among males and children aged < 5 years
- The reported prevalence of a neutrophil count < 1.0 × 109/L:2
Causes of Isolated Neutropenia
- Mechanistic:
- Decreased production: marrow failure, nutritional deficiencies, congenital syndromes, chemotherapy.
- Increased destruction: autoimmune neutropenia, drug-induced, viral infections.
- Sequestration: hypersplenism, margination in sepsis.
- Acquired transient neutropenia:
- Infection-related neutropenia:
- Causes include:
- Viral – the most common infectious cause of transient neutropenia:
- Respiratory viruses:
- Influenza
- Parainfluenza
- Respiratory syncytial virus (RSV)
- Adenovirus
- Rhinovirus
- SARS-CoV-2
- Systemic viral illnesses:
- Measles
- Rubella
- Varicella
- Cytomegalovirus (CMV)
- Epstein–Barr virus (EBV/mononucleosis)
- Hepatitis viruses
- Parvovirus B19
- HIV (acute infection)
- Respiratory viruses:
- Bacterial – less common, but transient neutropenia can occur, often from severe sepsis or overwhelming infection where bone marrow reserves are depleted:
- Typhoid fever (Salmonella typhi)
- Brucellosis
- Tuberculosis (occasionally)
- Some rickettsial infections (e.g., Rocky Mountain spotted fever, ehrlichiosis, anaplasmosis – these can cause significant transient or even prolonged neutropenia).
- Protozoal
- Viral – the most common infectious cause of transient neutropenia:
- Clinical Features of infection-related neutropenia:
- Neutropenia is usually mild to moderate.
- Onset coincides with acute illness and resolves in days to a couple of weeks.
- Severe, prolonged neutropenia should prompt evaluation for congenital, autoimmune, or drug-induced causes rather than infection alone.
- Key Points:
- Viral infections = most frequent cause of transient neutropenia.
- Bacterial/rickettsial infections can also cause it, sometimes more profoundly.
- Always consider the clinical context; febrile patient with neutropenia may still be at risk of serious bacterial infection, even if the neutropenia is “transient.”
- Causes include:
- Drug-induced neutropenia:
- Common agents include:
- Antiseizure medications:
- Carbamazepine
- Valproate
- Phenytoin
- Ethosuximide
- Trimethadione
- Antimicrobials:
- Sulfonamides
- Vancomycin
- Penicillins
- Trimethoprim/sulfamethoxazole
- Chloramphenicol*
- Cefepime
- Ciprofloxacin
- Meropenem
- Linezolid
- Metronidazole
- Tobramycin
- Valganciclovir
- Amoxicillin
- Antipsychotics:
- Clozapine
- Olanzapine
- Phenothiazines
- Tricyclic agents
- Anti-inflammatory and analgesics:
- Ibuprofen
- Diclofenac
- Sulfasalazine
- Gold salts
- Levamisole
- Penicillamine
- Metamizole (dipyrone)
- Antithyroid medications:
- Methimazole
- Thiamazole
- Propylthiouracil
- Diuretics
- Antiseizure medications:
- Overview:
- Patterns:
- Idiosyncratic/agranulocytosis (rare, unpredictable, immune-mediated or toxic to myeloid precursors).
- Dose-dependent marrow suppression (predictable, usually with cytotoxic/antimetabolite drugs).
- Timing: Often appears 1–6 months after starting the drug (idiosyncratic), or sooner with high-dose chemotherapy.
- Recovery: Neutrophil counts usually return to normal within 1–3 weeks after drug discontinuation.
- Patterns:
- Mechanisms:
- Direct bone marrow toxicity (chemotherapy, chloramphenicol).
- Immune-mediated destruction of neutrophils (antithyroid drugs, antibiotics, clozapine).
- Suppression of granulopoiesis (linezolid, interferon).
- Clinical Relevance:
- Can present with febrile neutropenia → high infection risk.
- Requires immediate drug discontinuation.
- Supportive therapy: G-CSF may hasten recovery in severe cases.
- Monitoring guidelines exist for high-risk drugs (e.g., weekly → monthly ANC with clozapine).
- Teaching Pearl: Always consider recent/new medications in any patient with new-onset neutropenia, especially if otherwise healthy.
- Common agents include:
- Neonatal alloimmune neutropenia:
- Caused by the passage of antineutrophil antibodies to the fetus via the placenta.
- Analogous to hemolytic disease of the newborn (from RBC antigens) and neonatal alloimmune thrombocytopenia.
- Clinical Features:
- Onset: typically within the first days of life.
- Neutropenia can be mild, moderate, or severe (ANC often < 0.5 × 10⁹/L in severe cases).
- Many infants are asymptomatic.
- Symptomatic cases: increased risk of bacterial infections (skin, umbilical stump, respiratory tract, sepsis).
- Unlike congenital neutropenia: normal bone marrow reserves and normal other lineages.
- Neonatal isoimmune neutropenia – caused by transplacental transfer of preexisting IgG antibodies from mothers with autoimmune neutropenia.
- Transfusion-related acute lung injury
- Infection-related neutropenia:
- Acquired chronic neutropenia:
- Immune-mediated causes:
- Primary autoimmune neutropenia (sometimes called chronic benign neutropenia of infancy/childhood):
- Definition:
- A benign, self-limited disorder in which autoantibodies (usually IgG) target neutrophil-specific antigens.
- Distinguished from secondary autoimmune neutropenia, which occurs in association with systemic autoimmune diseases (e.g., SLE, rheumatoid arthritis, autoimmune lymphoproliferative syndrome).
- Epidemiology:
- Most common cause of chronic neutropenia in children.
- Onset: typically between 6 months and 3 years.
- Estimated incidence: ~1 in 100,000 children.
- More common in females.
- Pathophysiology:
- Autoantibodies directed against human neutrophil antigens (HNAs) (especially HNA-1a, HNA-1b, HNA-2, HNA-4, HNA-5).
- Antibodies opsonize neutrophils → enhanced clearance by spleen and liver.
- Bone marrow shows normal or increased granulopoiesis, distinguishing it from marrow failure syndromes.
- Clinical Features:
- Neutropenia is usually moderate to severe (ANC <0.5 × 10⁹/L common).
- Despite low counts, serious infections are rare.
- Most children have only mild skin or mucosal infections (otitis media, gingivitis, sinusitis).
- Growth and development are otherwise normal.
- Diagnosis:
- CBC: isolated neutropenia; other lineages normal.
- Bone marrow: normal or increased myeloid precursors.
- Anti-neutrophil antibody testing (detection is challenging; false negatives are common, so diagnosis is often clinical).
- Exclude congenital neutropenias, cyclic neutropenia, marrow failure, or drug-induced causes.
- Natural History:
- Typically self-resolving within 1–3 years.
- Most children normalize neutrophil counts by age 4–5.
- Rarely persists beyond early childhood.
- Management:
- Observation and reassurance for most cases.
- Prompt treatment of infections with antibiotics.
- G-CSF (filgrastim) reserved for severe, recurrent, or life-threatening infections.
- Routine prophylactic antibiotics generally not needed.
- Live vaccines can usually be given if the child is otherwise well.
- Key Take-Home:
- Primary AIN = benign, self-limited, antibody-mediated neutropenia of infancy.
- Despite severe ANC depression, children usually remain healthy.
- Distinguishing feature: clinical course is mild, unlike congenital neutropenias or secondary autoimmune neutropenia.
- Definition:
- Secondary autoimmune neutropenia – associated with other autoimmune diseases:
- Definition:
- Neutropenia caused by autoantibodies directed against neutrophils, arising in the setting of another underlying disorder.
- Distinguished from primary AIN of infancy/childhood, which occurs in otherwise healthy young children and resolves spontaneously.
- Epidemiology:
- Much less common than primary AIN.
- Can present at any age, often in older children, adolescents, or adults.
- Reflects the underlying autoimmune or systemic disease rather than a benign, self-limited condition.
- Associated Conditions:
- Secondary AIN is most often linked to:
- Systemic autoimmune diseases
- Systemic lupus erythematosus (SLE)
- Rheumatoid arthritis / Felty’s syndrome
- Sjögren’s syndrome
- Autoimmune lymphoproliferative syndrome (ALPS)
- Other hematologic/immune disorders
- Chronic lymphocytic leukemia (CLL)
- Large granular lymphocyte (LGL) leukemia
- Common variable immunodeficiency (CVID)
- Post–allogeneic stem cell or solid organ transplantation
- Drug-induced autoimmunity (e.g., hydralazine, procainamide, some antithyroid drugs).
- Pathophysiology:
- Production of autoantibodies to neutrophil-specific antigens (HNAs), leading to:
- Peripheral destruction of neutrophils.
- Possible suppression of marrow granulopoiesis.
- Bone marrow: usually shows normal or increased precursors, unless coexisting marrow suppression.
- Clinical Features:
- Neutropenia may be persistent or intermittent.
- Severity varies: mild to severe (ANC <0.5 × 10⁹/L).
- Infection risk depends on severity, but higher than in primary AIN.
- Often accompanied by other autoimmune cytopenias (anemia, thrombocytopenia → Evans syndrome).
- Clinical clues: systemic autoimmune symptoms (rash, arthritis, lymphadenopathy, splenomegaly).
- Diagnosis:
- CBC: isolated or combined cytopenias.
- Autoantibody testing (anti-neutrophil antibodies) → limited sensitivity, but supportive if positive.
- Evaluate for underlying autoimmune or lymphoproliferative disease.
- Bone marrow: usually normal or hypercellular granulopoiesis.
- Management:
- Treat underlying disease (e.g., SLE, RA, CLL).
- Infection management: prompt antibiotics for febrile neutropenia.
- G-CSF (filgrastim): effective for raising counts, often used in symptomatic or severe neutropenia.
- Immunosuppression: corticosteroids, rituximab, or other agents in refractory cases.
- Long-term management depends on the associated condition.
- Key Take-Home:
- Secondary AIN is a manifestation of systemic autoimmune or lymphoproliferative disease.
- Unlike benign primary AIN of infancy, it requires investigation of the underlying disorder and sometimes immunosuppressive therapy.
- Definition:
- Primary autoimmune neutropenia (sometimes called chronic benign neutropenia of infancy/childhood):
- Chronic idiopathic/benign neutropenia:
- Definition:
- A condition characterized by persistent neutropenia without an identifiable cause after appropriate evaluation.
- “Benign” reflects the fact that most affected individuals do not experience severe or recurrent infections despite low absolute neutrophil counts (ANC).
- Often overlaps with the entity called chronic benign neutropenia of childhood or benign ethnic neutropenia (in certain populations).
- Epidemiology:
- Can occur in both children and adults.
- In children: sometimes considered a variant of primary autoimmune neutropenia, but many cases lack detectable antibodies.3
- In adults: more common in women (F:M ~8:1), usually discovered incidentally.
- Clinical Features:
- Isolated neutropenia (ANC often 0.5–1.5 × 10⁹/L, sometimes lower).
- Other blood counts normal.
- Most patients are asymptomatic.
- Infections, if present, are generally mild (skin, upper respiratory tract).
- No systemic features (e.g., no splenomegaly, lymphadenopathy, or autoimmune disease).
- Diagnosis:
- CBC: persistent neutropenia on multiple occasions (≥3 months).
- Bone marrow: typically normal granulopoiesis.
- Exclude other causes:
- Congenital neutropenias
- Autoimmune neutropenia
- Secondary causes (drugs, infections, systemic disease, marrow disorders)
- Anti-neutrophil antibody testing may be negative.
- Pathophysiology:
- In many cases, mechanism unclear (hence idiopathic).
- May involve subtle immune-mediated destruction, increased margination/sequestration, or altered marrow release.
- Natural History:
- Usually stable over years.
- In children, often resolves spontaneously within a few years.
- In adults, tends to persist but remains clinically benign.
- Management:
- Observation only in most cases.
- Reassurance: risk of severe infection is low, even with ANC <0.5 × 10⁹/L in some cases.
- Prompt treatment of infections as they arise.
- G-CSF or immunosuppressive therapy rarely required, except for recurrent/severe infections.
- No contraindication to vaccines.
- Key Take Home:
- Chronic idiopathic/benign neutropenia is a diagnosis of exclusion.
- Despite low counts, the majority of patients remain healthy and infection-free.
- Recognizing it prevents unnecessary investigations or treatments.
- Definition:
- Immune-mediated causes:
- Congenital Neutropenia:
- Not associated with increased risk of infections:
- Constitutional neutropenia (Duffy-null associated neutrophil count):
- Definition:
- A form of lifelong, physiologic neutropenia characterized by a chronically low ANC (often 1.0–1.5 × 10⁹/L, sometimes <1.0) without increased infection risk.
- Strongly associated with homozygosity for the Duffy-null allele (DARC/ACKR1 gene), which abolishes Duffy antigen expression on red blood cells.
- Epidemiology:
- Prevalence:
- Up to 25–50% in individuals of sub-Saharan African descent.
- ~10–15% in Middle Eastern populations.
- Rare in Europeans and Asians.
- The Duffy-null phenotype (Fy[a–, b–]) provides protection against Plasmodium vivax malaria, explaining its high frequency in endemic regions.
- Prevalence:
- Pathophysiology:
- Caused by a SNP in the ACKR1 gene promoter (–46C>C, rs2814778) → loss of Duffy antigen expression on RBCs.
- Mechanism of neutropenia is not fully understood, but thought to involve:
- Altered neutrophil trafficking and margination.
- Reduced release from marrow pools.
- Importantly: granulopoiesis is normal, and neutrophil function is intact
- Clinical Features:
- ANC persistently below “normal” lab ranges (often 0.8–1.5 × 10⁹/L).
- Asymptomatic: no increased risk of bacterial or fungal infections.
- Other blood counts are normal.
- Discovered incidentally on routine CBCs.
- Diagnosis:
- Lifelong stable neutropenia in the absence of infections or systemic disease.
- Ethnic background and family history often provide clues.
- Genetic testing for ACKR1 polymorphism can confirm diagnosis (not always necessary clinically).
- Bone marrow: normal.
- Clinical Significance:
- Benign condition — reassurance is key.
- Important to distinguish from pathologic neutropenia to avoid unnecessary work-up or discontinuation of needed drugs.
- ANC thresholds for “neutropenia” in clinical trials and chemotherapy risk stratification may need adjustment in affected populations (otherwise patients may be misclassified as high risk).
- Key Take-Home:
- Duffy-null associated neutrophil count = normal variant of neutrophil physiology.
- No treatment required.
- Clinical pearl: not all neutropenia is pathologic — consider this especially in individuals of African or Middle Eastern descent.
- Definition:
- Benign familial neutropenia:
- Similar to constitutional neutropenia, clearly hereditary but not linked to a particular ethnic group.
- A diagnosis of exclusion but can be confidently made when multiple healthy relatives across generations have stable, mild neutropenia without infections.
- Constitutional neutropenia (Duffy-null associated neutrophil count):
- Typically associated with infections:
- Cyclic neutropenia:
- Definition:
- A rare inherited bone marrow failure syndrome characterized by regular, periodic oscillations in neutrophil counts, with severe neutropenia recurring every ~21 days.
- During neutropenic nadirs, patients are predisposed to infections; between episodes, counts are near-normal.
- Epidemiology:
- Incidence: ~1 per 1 million.
- Inherited in an autosomal dominant pattern, usually caused by heterozygous mutations in ELANE (neutrophil elastase gene).
- Onset: typically in infancy or early childhood, but can present later.
- Pathophysiology:
- Mutant ELANE protein causes misfolded neutrophil elastase, leading to increased apoptosis of myeloid precursors.
- Results in periodic “stalling” of granulopoiesis, creating cyclic drops in ANC.
- Oscillations also affect monocytes, reticulocytes, and platelets to a lesser extent.
- Clinical Features:
- Cycle length: ~21 days (range 14–35).
- Nadir ANC: often <0.2 × 10⁹/L, lasting 3–5 days.
- Symptoms cluster during neutropenic periods:
- Fever, malaise.
- Aphthous ulcers, pharyngitis, gingivitis, periodontitis.
- Recurrent skin infections, cellulitis, lymphadenitis.
- Less commonly, sepsis.
- Between nadirs, patients are usually well.
- Diagnosis:
- Requires serial CBCs (2–3 times/week for 6–8 weeks) to document cyclic pattern.
- Bone marrow: shows “maturation arrest” at the myelocyte/promyelocyte stage during nadir; recovers in between.
- Genetic testing: ELANE mutations in most cases.
- Management:
- G-CSF (filgrastim): dramatically reduces infection risk, shortens nadir duration, increases ANC.
- Oral hygiene and infection prevention critical (dental complications are common).
- Prompt antibiotics during febrile episodes.
- Prognosis: good with G-CSF; unlike severe congenital neutropenia, cyclic neutropenia carries a very low risk of MDS/AML transformation.
- Key Teaching Pearls:
- Suspect cyclic neutropenia in children with recurrent fevers, mouth ulcers, and infections at regular intervals.
- Diagnosis requires serial counts, not a single CBC.
- G-CSF is highly effective and life-changing for these patients.
- Definition:
- Severe congenital neutropenia (SCN):
- Definition:
- A group of rare inherited bone marrow failure syndromes characterized by persistent, severe neutropenia (ANC usually < 0.5 × 10⁹/L) present from infancy.
- Distinguished from benign/idiopathic neutropenias by early onset, high infection risk, and progression risk to MDS/AML.
- Epidemiology:
- Incidence: ~1–2 per 200,000 live births.
- Onset: infancy (first few months of life).
- Both sexes affected.
- Genetics:
- Most commonly due to ELANE mutations (encoding neutrophil elastase) → 40–60% of cases.
- Other implicated genes:
- HAX1 (Kostmann disease, autosomal recessive, often with neurologic features).
- G6PC3, JAGN1, WAS, CSF3R, VPS45, others.
- Inheritance: autosomal dominant, recessive, or sporadic.
- Pathophysiology:
- Defective granulopoiesis with maturation arrest at the promyelocyte/myelocyte stage in bone marrow.
- Profound lifelong neutropenia → high susceptibility to infections.
- With time and G-CSF exposure, increased risk of somatic CSF3R mutations and evolution to MDS/AML.
- Clinical Features:
- Infants present with life-threatening infections:
- Omphalitis, skin abscesses, pneumonia, sepsis.
- Recurrent oral ulcers, gingivitis, periodontitis.
- ANC typically <0.2–0.5 × 10⁹/L.
- Other cytopenias usually absent at baseline, but can appear with clonal evolution.
- Infants present with life-threatening infections:
- Diagnosis:
- CBC: persistent, severe neutropenia.
- Bone marrow: cellular with granulocytic maturation arrest.
- Genetic testing confirms subtype.
- Must exclude acquired causes (aplastic anemia, autoimmune, infection, drugs).
- Management:
- G-CSF (filgrastim): cornerstone therapy, raises ANC, reduces infection risk, allows survival into adulthood.
- Antibiotics: aggressive management of infections.
- HSCT: only curative option, considered in G-CSF–refractory patients or those with clonal evolution/MDS/AML.
- Monitoring: lifelong surveillance with annual marrow exams + cytogenetics (looking for monosomy 7, CSF3R mutations, RUNX1 mutations).
- Prognosis:
- With G-CSF, survival dramatically improved.
- However, 20–30% lifetime risk of MDS/AML.
- Risk higher in poor G-CSF responders (high dose requirement >8 μg/kg/day).
- Key Teaching Pearls:
- SCN = early-onset, severe neutropenia with maturation arrest.
- Always distinguish from benign neutropenia of childhood.
- G-CSF is life-saving, but patients need long-term monitoring for leukemic transformation.
- Definition:
- Neutropenia may also be associated with complex phenotypes, typically diagnosed in childhood, for example:
- Shwachman-Diamond syndrome
- Reticular dysgenesis
- Barth syndrome
- Glycogen storage disease type 1b
- Glucose-6-phosphate catalytic subunit 3 syndrome
- Chediak-Higashi syndrome
- Cohen syndrome
- Griscelli syndrome type II
- Hermansky-Pudlak syndrome type II
- P14 deficiency
- Cartilage-hair hypoplasia
- WHIM syndrome
- Hyper-IgM syndrome
- Wiskott-Aldrich syndrome
- Cyclic neutropenia:
- Not associated with increased risk of infections:
Initial Clinical Approach
The first priority is to identify patients at immediate risk. Severe or febrile neutropenia is a medical emergency and must be treated with empiric antibiotics. In less urgent cases, classify the neutropenia (severity, duration, acquired vs congenital, dynamics) to narrow the differential. Careful history and exam provide important clues.
- Urgency: febrile + severe neutropenia = emergency.
- History: infections, drugs, autoimmune disease, family history, ethnicity, nutrition.
- Exam: oral ulcers, gingivitis, lymphadenopathy, splenomegaly, systemic clues.
Stepwise approach:
- Confirm and Characterize the Neutropenia:
- Repeat CBC with differential to rule out lab error or transient fluctuation.
- Review prior CBCs → chronic vs new onset.
- Determine severity:
- Mild: ANC 1.0–1.5 × 10⁹/L
- Moderate: ANC 0.5–1.0 × 10⁹/L
- Severe: ANC <0.5 × 10⁹/L
- Assess Clinical Context:
- Symptoms/signs: fevers, infections, mouth ulcers, gingivitis, skin infections.
- Physical exam: oral cavity, lymphadenopathy, hepatosplenomegaly, rash.
- History clues:
- Duration: acute/transient vs chronic (>3 months).
- Family history of cytopenias or early deaths.
- Ethnicity (consider constitutional neutropenia / Duffy-null).
- Drug exposure (antibiotics, antithyroid, anticonvulsants, clozapine, chemotherapy).
- Recent or current infections (especially viral).
- Autoimmune symptoms (rash, arthritis, cytopenias).
- Nutritional history (B12, folate, copper).
- Initial Laboratory Evaluation (see next section):
- Classify by Pattern:
- Transient (usually post-infectious or drug-related).
- Chronic (>3 months):
- Benign variants: constitutional (Duffy-null), benign familial.
- Autoimmune: primary in children, secondary in SLE/RA/CLL/ALPS.
- Congenital syndromes: severe congenital neutropenia, cyclic neutropenia, Shwachman-Diamond, GATA2 deficiency.
- Marrow failure / infiltration: aplastic anemia, MDS, leukemia, lymphoma.
- Key Teaching Pearls:
- Not all neutropenia is dangerous → constitutional and benign causes are common.
- Always ask: acute vs chronic? mild vs severe? symptomatic vs asymptomatic?
- The clinical story (infection history, systemic disease, family history, ethnicity, drug exposure) is often more important than the count itself.
Laboratory Evaluation
- General considerations:
- CBC with differential to confirm ANC and assess other cytopenias. Perform serial CBC panels over a period of days or weeks to establish a trend in neutrophil count and chronicity.
- Peripheral smear for blasts, dysplasia, or toxic changes.
- Nutritional labs (B12, folate, copper).
- Infectious workup (HIV, hepatitis, EBV, CMV, parvovirus).
- Autoimmune markers (ANA, rheumatoid factor, others as indicated).
- Bone marrow aspirate/biopsy:
- Indications include:
- Severe or persistent neutropenia of unclear cause.
- Additional cytopenias.
- Abnormal smear (blasts, dysplasia).
- Poor response to G-CSF or unexplained infections.
- Indications include:
- Evaluation of suspected acquired neutropenia:
- If suspected, obtain serology and cultures for:
- Viruses (Ebstein-Barr virus, cytomegalovirus, HIV, influenza) that may cause transient neutropenia
- Mycobacterial, rickettsial, protozoan, and other bacterial infections
- Evaluate for immune function disorders and autoimmune diseases such as RA or SLE:
- Rheumatoid factor
- Antinuclear antibody (ANA)
- Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)
- Quantitative immunoglobulin (Ig)G, IgA, and IgM.
- Antineutrophil antibodies can be useful in the pediatric age group. The use of this test in diagnosing adults with autoimmune neutropenia is controversial, and there is a high rate of false positives and false negatives.
- Consider peripheral blood flow cytometry to identify:
- Leukemia and lymphoma markers
- Large granular lymphocyte (LGL) leukemia by detection of a clonal T-cell receptor (TCR) rearrangement or activated natural killer cells
- If pancytopenia is present, measure vitamin B12 and folate levels for evidence of deficiencies.
- If suspected, obtain serology and cultures for:
- Evaluation of suspected congenital neutropenia:
- Assess ethnicity. In asymptomatic persons of African descent with mild neutropenia, extensive work-up is not necessary.
- If using genetic sequencing to confirm a diagnosis of severe congenital neutropenia (SCN), consider sequencing ELANE first as pathogenic variants in ELANE are most common, unless there are family history, physical examination, or laboratory testing results that suggest another diagnosis.
- If cyclic neutropenia is suspected, perform serial complete blood counts with differential ≥ twice weekly for a minimum of 4-6 weeks to establish a cyclic pattern and assess for ELANE gene mutation.
- If SCN is suspected, assess for ELANE and HAX1 gene variants.
- Consider a genetic analysis to identify complex disorders associated with neutropenia.
Management Principles
- Observation for mild, asymptomatic cases.
- Stop offending drugs when suspected.
- Treat underlying infection or condition.
- Autoimmune neutropenia: steroids, immunosuppressives, or rituximab in refractory cases.
- G-CSF for severe or chronic symptomatic neutropenia.
- Prophylaxis: antimicrobials or growth factors in high-risk patients.
The Diagnostic Blind Spot: Isolated Neutropenia
For isolated neutropenia in adults, the evaluation often boils down to a somewhat blunt sequence: repeat CBCs, review of medications, infectious/autoimmune screening, sometimes nutritional labs, and ultimately bone marrow examination if no cause emerges. Beyond this, there are relatively few specialized, validated, widely available tools. Molecular/genetic testing exists (e.g., congenital neutropenia syndromes, clonal hematopoiesis), but it’s rarely applied in routine adult cases unless suspicion is high. Functional assays (e.g., granulocyte antibody testing, marrow reserve tests like glucocorticoid stimulation) are niche and inconsistently used.
In contrast, for anemia and thrombocytopenia, we have:
- Thrombocytopenia: platelet function testing, flow cytometry, anti-platelet antibody testing (limited utility but present), increasingly precise bone marrow morphologic and molecular correlation (e.g., clonal hematopoiesis, MDS panels), not to mention advanced imaging for splenic sequestration.
- Anemia: sophisticated iron studies, hemoglobin electrophoresis, reticulocyte indices, erythropoietin, soluble transferrin receptor, even hepcidin research assays.
Why the discrepancy?
- Physiology:
- Erythrocytes and platelets have clear quantitative and qualitative laboratory “handles” (indices, morphology, function assays).
- Neutrophils are more “binary”: present or absent. Their function is complex (chemotaxis, phagocytosis, oxidative burst), but routine clinical tests for these are cumbersome and largely confined to immunology/inborn error labs.
- Disease distribution:
- Isolated neutropenia in adults is relatively uncommon compared to anemia and thrombocytopenia, so diagnostic tools haven’t been pushed to the same degree by demand.
- Therapeutics drive diagnostics:
- In anemia, the explosion of iron therapies, ESAs, and gene therapies has created incentives for better diagnostic granularity.
- In thrombocytopenia, the need to distinguish ITP from marrow failure, TTP, etc., has driven tool development.
- For neutropenia, outside of G-CSF and infection prophylaxis, therapeutic options remain limited, so the diagnostic armamentarium has lagged.
Bottom line
Our workup for adult isolated neutropenia feels outdated, algorithmic, and not nearly as nuanced as for anemia or thrombocytopenia. It’s partly because neutrophils are harder to assay in meaningful ways, partly because demand is lower, and partly because therapeutic innovation hasn’t forced diagnostic innovation.
Summary
Neutropenia should be classified by severity, duration, etiology, and dynamics. This framework helps distinguish life-threatening emergencies from benign, chronic, or transient forms, and guides rational evaluation and management.
References
- Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014 Aug 21;124(8):1251-8.
- Boxer LA. How to approach neutropenia. Hematology Am Soc Hematol Educ Program. 2012;2012:174-82.
- Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013 Jul;50(3):198-206.
- Bartels M, Murphy K, Rieter E, Bruin M. Understanding chronic neutropenia: life is short. Br J Haematol. 2016 Jan;172(2):157-69.
