Postscript
Introduction
- Folate is one of the B vitamins (vitamin B9).
- Humans cannot make folate. It is an essential nutrient, required in the diet.
- Folates act as one-carbon donors in the synthesis of DNA, RNA and certain neurotransmitters, phospholipids, and hormones.
- Folate deficiency may cause anemia and neural tube defects in pregnancy.
- Folate deficiency is commonly tested for in the context of macrocytic anemia.
- Folate deficiency is now rare due to fortification of foods in many countries.
- Folate deficiency has also been hypothesized to affect other conditions including dementia, delirium, peripheral neuropathy, depression, cancer, and cardiovascular disease.
Dietary fortification
- In 1996, the US Food and Drug Administration (FDA) mandated that all foods with processed grains be supplemented by the manufacturers with 140 g of folate per 100 g beginning in January 1998.
- In 1998 Canada also mandated similar fortification.
- The purpose of the American and Canadian mandates was specifically to provide women of child-bearing age additional folate in the hopes of lowering the risk for neural tube defects in their children.
- The mandatory fortification of grain products:
- Have added about 0.1 mg folate to the daily intake of an adult. Even if a patient has no other source of folate intake, a daily intake of 0.1 mg folic acid is more than enough to prevent folate deficiency anemia.
- Markedly reduced the prevalence of folate deficiency and increased the mean serum folate of the population.
Definitions
- The terms “folic acid” and “folate” often are used interchangeably. However, some differences are recognized:
- Folate:
- The term encompassing all the different biologically active forms of the vitamin. Some include folic acid in this category, others restrict the use of the term folates to natural food sources.
- Natural folate can be found in foods such as leafy green vegetables, citrus fruits, and beans.
- 90% of the folate found in foods are polyglutamates.
- Naturally-occurring folate is a water-soluble molecule that exists physiologically as tetrahydrofolate (THF, the active form) and methyltetrahydrofolate (MTHF, primary form found in blood)
- Bioavailability of food folates is, on average, about 50% lower than folic acid.
- Folic acid:
- The synthetic form used in:
- Supplements
- Fortified foods
- For treatment
- Chemically stable (more stable than folate) and fully oxidized pro-vitamin. Its biological activity depends on the action of dihydrofolate reductase (DHFR) enzyme. In order to fulfill a physiological function by entering the folate cycles, FA must first be reduced by DHFR to dihydrofolate (DHF), and then to tetrahydrofolate (THF), before it is converted to the biologically active 5-MTHF.
- Always exists as a monoglutamate.
- The synthetic form used in:
- Folate:
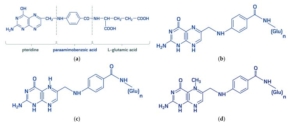
Chemical structure of (a) folic acid and its metabolically active derivates: (b) dihydrofolate, (c) tetrahydrofolic acid, (d) 5-methyltetrahydrofolate. “Folates” is a generic term encompassing folic acid and its derivatives-dihydro-, tetrahydro-, methyl-, formyl-compounds possessing metabolic activity. All folates are inherently conjugated with para-aminobenzoyl-glutamate as mono-, di-, tri-, and polyglutamates. Molecules 2021, 26, 3731.
- Folate deficiency:
- There is no clear consensus on the level of serum folate that indicates deficiency.
- Most clinicians define folate deficiency as serum folate lower than 7 nmol/l (3 mcg/l) because the risk of megaloblastic anaemia greatly increases below this level.
Epidemiology
- Prior to mandatory folic acid fortification in the United States, the prevalence of folate deficiency was estimated to be between 3% and 16%.
- National Health and Nutrition Examination Survey (NHANES) data indicate a substantial decrease in folate deficiency from 16% to 0.5% before and after fortification, respectively.1
- Cohort of 2093 serum folate levels were obtained on 1944 inpatients and emergency department patients:
- Of the 2093 serum folate levels:
- 2 were deficient (0.1%)
- 7 were low-normal (0.3%)
- 1487 were normal (71.1%)
- 597 were high (28.5%)
- Total charge and charge per deficient result as $149,545 and $74,772, respectively.
- Of the 2093 serum folate levels:
- Cohort of 937 cancer inpatients had 1065 tests performed during 2017:
- Among all folate testing instances, 96% co-occurred with testing for vitamin B12 deficiency
- 7.0% had folate deficiency.
- 3.5% had isolated vitamin B12 deficiency.
- 0.3% of all co-testing instances were simultaneously deficient for both micronutrients.
- Cohort of patients with 2154 serum folate assayed in 2001:
- 11 (0.5%) were low (SF of 3.9-6.6 nmol/L).
- Only one case had a high mean corpuscular volume (MCV)
- In none of the patients seen at SBGH after 1998, could the anemia or macrocytosis be attributed to folate deficiency.
- In another study, red blood cell folate was ordered at 3 hospitals that predominately service the needs of indigent patients:2
- Using a red blood cell folate cutoff concentration of 160 ng/mL (363.6 nmol/L), the combined incidence of folate deficiency decreased from 4.8% in 1997 to 0.6% in 2004.
- At a cutoff of 94 ng/mL (213 nmol/L), the incidence went from 0.98% to 0.09% in 1997 and 2004, respectively.
- Even when the folate concentration was found to be low, the majority of these subjects did not have macrocytosis.
Physiology
- Folate encompasses a family of over 150 biologically and structurally related compounds that differ in three respects:
- Additional glutamate residues may be conjugated to one another via glutamyl bonds at the end of the molecule, forming a polyglutamate tail.
- The pteridine ring may be reduced to give either 7,8-dihydrofolate (DHF) or the coenzymatically active 5,6,7,8-tetrahydrofolate (THF) form.
- The reduced forms can be substituted with covalently bonded one-carbon units at the N-5 or N-10 nitrogen positions.
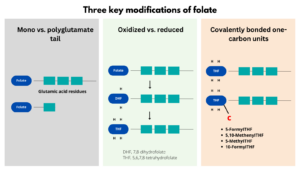
THF is the metabolically active form of folate which can carry various one-carbon groups at the N-5 and N-10 positions, giving rise to numerous folate coenzymes with diverse biological functions.
- THF polyglutamates are the form of the vitamin present in cells and in food from natural source. Folic acid is the synthetic form used in supplements, fortified foods, and for treatment.
- Folate is produced by microorganisms and plants, which account for dietary sources including vegetables and fruits.
- Absorption:
- Folate is most efficiently absorbed in the proximal jejunum where its bioavailability depends upon the host’s capacity to hydrolyze the polyglutamate chain.
- Natural folates:
- Dietary folate exists in as THF polyglutamates, which must be hydrolyzed to THF monoglutamates in the gastrointestinal tract before absorption across the intestinal epithelium by the proton-coupled folate transporter (PCFT).
- In enterocytes, THF monoglutamate converted to 5-methyl-THF, which is then released into the circulation
- Folic acid:
- Exists as a monoglutamate, thus no need for hydrolysis.
- Absorbed across the intestinal epithelium by the proton-coupled folate transporter (PCFT).
- In enterocytes and hepatocytes absorbed folic acid at first is reduced by dihydrofolate reductase to dihydrofolate (DHF) and tetrahydrofolate (THF).
- THF is then converted to 5-methyl-THF, which is released into the circulation.
- Circulating folate:
- After enterocyte uptake, folate is metabolized to 5-methyl-THF and released into the portal circulation for storage in the liver and subsequent redistribution to peripheral tissues.
- 5-methyl-THF is the most prevalent physiological form of folate in systemic circulation.
- Intracellular folate:
- Cellular folate transport occurs primarily via two pathways:
- Reduced folate carrier (RFC):
- A transmembrane protein which uses a bidirectional anion-exchange mechanism to transfer hydrophilic folates across the cell membrane.
- Responsible for mediating uptake of serum 5-methyl-THF across most tissues in the body.
- Folate receptors (FRs):
- FRs bind folate with high affinity and mediate cellular uptake by an endocytotic mechanism. FRs exist in three isoforms which have tissue-specific expression:
- FR-alpha and FR-beta are membrane bound receptors.
- FR-gamma is nonmembrane bound and found only in hematopoietic tissues.
- FRs bind folate with high affinity and mediate cellular uptake by an endocytotic mechanism. FRs exist in three isoforms which have tissue-specific expression:
- Reduced folate carrier (RFC):
- Folate is polyglutamated within peripheral tissues to:
- Ensure its intracellular retention.
- Enhance the affinity of folate coenzymes for several folate-dependent enzymes.
- Inside cells, folate functions in one-carbon metabolism, required for DNA synthesis, repair, and methylation, and normal cell proliferation:
- Folate derivatives participate in biosynthetic reactions involving transfers of groups containing a single carbon atom.3 These functional units are essential components in the metabolism of the amino acids glycine, serine, methionine, and histidine, and the biosynthesis of purines and pyrimidines.
- Tetrahydrofolate (THF), the reduced form of folic acid, is the primary carrier of key components of the cellular machinery involving the mobilization and utilization of single-carbon functional groups at the methyl, methylene and formyl oxidation levels.
- Tetrahydrofolate derivatives serve as useful storage units of these functional groups that are not detrimental to the host organism, but are poised for reactivity when recruited by biosynthetic enzymes.
- Cellular folate transport occurs primarily via two pathways:
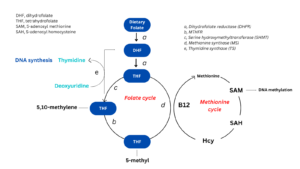
In enterocytes and hepatocytes absorbed folic acid at first is reduced by dihydrofolate reductase to dihydrofolate (DHF) and tetrahydrofolate (THF). THF is converted to 5,10- methylene-THF by pyridoxine-dependent enzyme serine hydroxymethyltransferase and then is reduced to 5-methyl-THF by methylenetetrahydrofolate reductase (MTHFR). THF and 5-methyl-THF are the most biologically active folates playing essential role in cellular one-carbonic metabolism.
Pathophysiology
- Since folate is required for DNA synthesis, the earliest signs of deficiency are seen in rapidly proliferating cells, such as those of the bone marrow and gastrointestinal tract.
- Severe folate deficiency can cause pancytopenia and megaloblastic anaemia.
- Sequence of events in folate deficiency:
- Sequential events in the blood:
- Decrease in plasma folate concentration.
- Increase in plasma homocysteine concentration
- Reduced RBC folate
- Morphological changes in blood and bone marrow:
- Megaloblastic changes in the bone marrow and other rapidly dividing tissues:
- Megaloblastic cells are abnormally large cells with large nuclei (characterized by a finely stippled lacy pattern of nuclear chromatin) and apparently normal cytoplasm, which give rise to the classic morphologic finding of nuclear-cytoplasmic dissociation.
- Hypersegmentation of nuclei in neutrophils or generation of micronuclei in lymphocytes, which are a biomarker of chromosome breakage or loss.
- Megaloblastic changes in the bone marrow and other rapidly dividing tissues:
- The incapacitation of erythroblast replication results in reduced and abnormal erythrocyte production leading to anemia and reduced oxygen-carrying capacity of the blood, which may lead to symptoms of weakness, fatigue, irritability, and shortness of breath.
- Sequential events in the blood:
Causes of folate deficiency
- Congenital:
- Mutations in the SLC46A1 (PCFT; HGNC 30521) gene (proton-coupled folate transporter deficiency)
- Acquired:
- Low intake of folate (most common cause of folate inadequacy) as seen in:
- Alcoholics
- Drug abusers
- Elderly
- Individuals of lower socioeconomic status (who do not consume high-folate-content foods)
- Poor absorption of folate (at the level of the jejunum) as occurs with:
- Surgical resection
- Celiac disease
- Tropical sprue
- Inflammatory bowel disease
- Short bowel syndrome
- Bacterial overgrowth
- Drugs such as:
- Alcohol
- Anticonvulsants
- Oral contraceptives
- Increased folate requirement as seen in:
- Pregnancy (rapid cell replication and growth of fetal, placental, and maternal tissue)
- Lactation
- Pediatric patients, rapid growth in adolescence
- Conditions associated with rapid cellular proliferation such as:
- Hemolysis
- Malignancy
- Exfoliative dermatitis
- Low intake of folate (most common cause of folate inadequacy) as seen in:
Clinical presentation
- May present with:
- Incidental finding of low serum folate levels.
- Symptoms of anemia including:
- Fatigue
- Shortness of breath on exertion
- Adverse outcomes in pregnancy, including:
- Neural tube defects
- Neural crest disorders (e.g., congenital heart defects)
- Fetal growth retardation
- Preterm delivery
- Neonatal folate deficiency
- Considering the time course of anemia development may also be helpful in clinically differentiating vitamin B12 and folate deficiency. In adults, folate stores may become exhausted after only 3–5 months, whereas vitamin B12 stores are more longstanding and thus a B12 deficiency may manifest after two to five years.
Diagnosis
- Suspect diagnosis in patient with:
- CBC and peripheral blood smear showing:
- Macrocytic anemia (+-/ leukopenia and/or thrombocytopenia)
- Presence of oval macrocytes.
- Hypersegmented neutrophils, defined as >5% of neutrophils with five or more lobes
- Hemolytic markers including:
- Increased:
- Lactate dehydrogenase
- Indirect bilirubin
- Decreased haptoglobin
- Increased:
- CBC and peripheral blood smear showing:
- Confirm diagnosis with:
- Measurement of serum folate:
- Earliest indicator of altered folate exposure.
- Reflects recent dietary intake (i.e., short-term status).
- Falsely normal results may occur due to postprandial increase (within 2 h) in non-fasting samples.
- Serial measurements may be required.
- Assays include:
- Microbiological assay – based on the ability of Lactobacillus rhamnosus to grow in the presence of folate monoglutamate.
- Competitive folate binding protein (FBP) assays using chemiluminescence or fluorescence detection system – most common assay used.
- LC-MS/M
- Measurement of red blood cell folate:
- Sensitive indicator of long term folate status.
- Represents the amount of folate that accumulates in RBCs during erythropoiesis, thereby reflecting folate status during the preceding 120 days.
- Not affected by recent dietary intake.
- Recent blood transfusion can lead to inaccurate results.
- Parallels liver concentrations (accounting for ;50% of total body folate) and is thus considered to reflect tissue folate stores.
- Measurement of plasma homocysteine:
- A sensitive functional biomarker of folate status.
- Inversely related to folate status (whether measured as serum or RBC folate).
- Also elevated in B12 and B6 deficiency, renal failure, hypothyroidism.
- There is no consensus on the reference range although most laboratories regard a concentration above 15 lmol/l as indicative of hyperhomocysteinemia.
- Measurement of serum folate:
- Bone marrow aspirate and biopsy rarely indicated; if performed may show:
- Hypercellularity
- Megaloblastic changes in erythroid lineage:
- Erythroid hyperplasia
- Increased pronormoblasts
- Nuclear-cytoplasmic asynchrony (finely dispersed nuclei and mature cytoplasm with hemoglobinization)
- Binucleated and multinucleated forms
- Nuclear budding
- Fragmented nuclei
- Megaloblastic changes in granulocytic lineage:
- Giantism of bands and metamyelocytes
- Nuclear hypersegmentation of mature granulocyte
- British Committee for Standards in Haematology 2014 guideline:
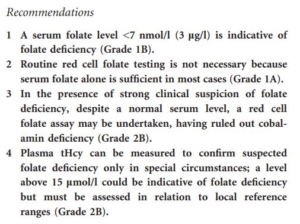
To order folate assay or not order
- As the result of fortification of grain products with folic acid, dietary folate deficiency has become very rare. Consequently, some authorities have stated there is no longer any justification in ordering folate assays to evaluate the folate status of the patient:
- “There are still some patients who may be folate deficient (very small premature babies before they are able to start taking grain products, pregnant women who are not taking multivitamins and who also avoid eating grain products, malnourished alcoholics or those with celiac disease or tropical sprue who are avoiding taking grain products). However, these patients could be easily identified by their medical history and could be placed on prophylactic folic acid therapy without testing for folate deficiency, as long as it is determined that their SB12 is normal.”4
- American Society for Clinical Pathology Choosing Wisely:
- Do not order red blood cell folate levels at all. In adults, consider folate supplementation instead of serum folate testing in patients with macrocytic anemia.
- Since 1998, when the U.S. and Canada mandated that foods with processed grains be fortified with folic acid, there has been a significant decline in the incidence of folate deficiency. For the rare patient suspected of having a folate deficiency, simply treating with folic acid is a more cost-effective approach than blood testing. While red blood cell folate levels have been used in the past as a surrogate for tissue folate levels or a marker for folate status over the lifetime of red blood cells, the result of this testing does not, in general, add to the clinical diagnosis or therapeutic plan.
- Not all authors agree with the above approach. Consider, for example:
- “The Choosing Wisely organization further indicated that providers could commence folate supplementation treatment if macrocytic anemia is suspected without testing for folate status. However, peer-reviewed reports of elevated folate-related risks and resurgence of cancers and other morbidities have multiplied. Recent data from the National Institutes of Health revealed a strong association between oversupplementation of folate and vitamin B12 deficiency anemia masking with concomitantly serious clinical implications, since folate is required to activate vitamin B12. The evidence provided above suggests the need for further investigation into the clinical utility of folate assessment.”5
Treatment
- British Committee for Standards in Haematology (BCSH) recommends treating folate disorders according to the British National Formulary schedules. The BNF has outlined the treatment of folate deficiency as follows:
- Folate deficient megaloblastic anaemia (due to dietary insufficiency, pregnancy or antiepileptics): 5 mg of folic acid daily is taken for 4 months, except in pregnancy where it is continued until term, and up to 15 mg daily for
4 months is suggested in malabsorptive states. - Chronic hemolytic states and renal dialysis: the prophylactic dose suggested is 5 mg daily to weekly, depending on the diet and rate of hemolysis.
- Pregnancy: the prophylactic dose suggested is 200–500 mcg daily.
- Folate deficient megaloblastic anaemia (due to dietary insufficiency, pregnancy or antiepileptics): 5 mg of folic acid daily is taken for 4 months, except in pregnancy where it is continued until term, and up to 15 mg daily for
- Folic acid metabolite levels (homocysteine and methylmalonic acid) reported to normalize 1-2 weeks after onset of folic acid replacement therapy.
- Full hematologic response should occur within 8 weeks.
