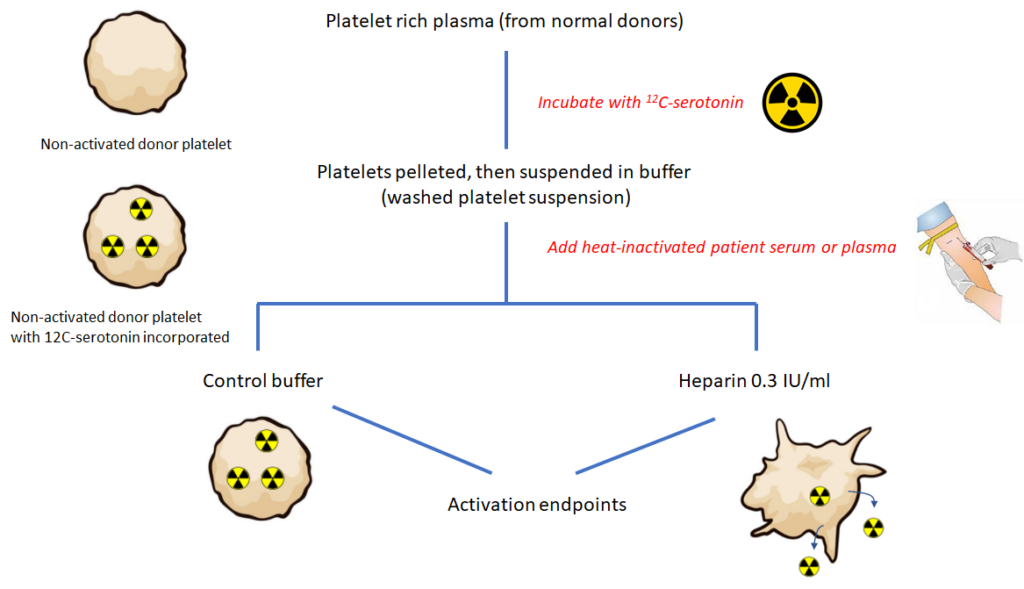
The SRA is a functional assay used to diagnose HIT. It identifies pathogenic IgG antibodies capable of binding and cross-linking platelet Fc gamma RIIA and triggering platelet activation. Test results are expressed as percentage of serotonin release compared to maximum release after detergent-induced platelet lysis (100%). Positive test is > 20% release at therapeutic heparin levels and < 20% release at supratherapeutic heparin levels. Specificity about 95%, but lower sensitivity reported compared with immunoassays.