Caplacizumab in TTP
Prev
1 / 0 Next
Prev
1 / 0 Next
Caplacizumab in thrombotic thrombocytopenic purpura (TTP)
Overview:
- A humanized, bivalent, variable-domain-only immunoglobulin fragment (Nanobody, Ablynx) directed to the A1 region of von Willebrand factor, which specifically targets the A1 domain of von Willebrand factor, preventing interaction with the platelet glycoprotein Ib-IX-V receptor (platelet-von Willebrand factor interactions) and the ensuing microvascular thrombosis in small arterioles and capillaries.
- Caplacizumab-yhdp (Cablivi) FDA approved in 2019 for treatment of adults with immune TTP in combination with plasma exchange and immunosuppressive therapy.
- Represents the first drug to receive a regulatory approval for the treatment of iTTP.
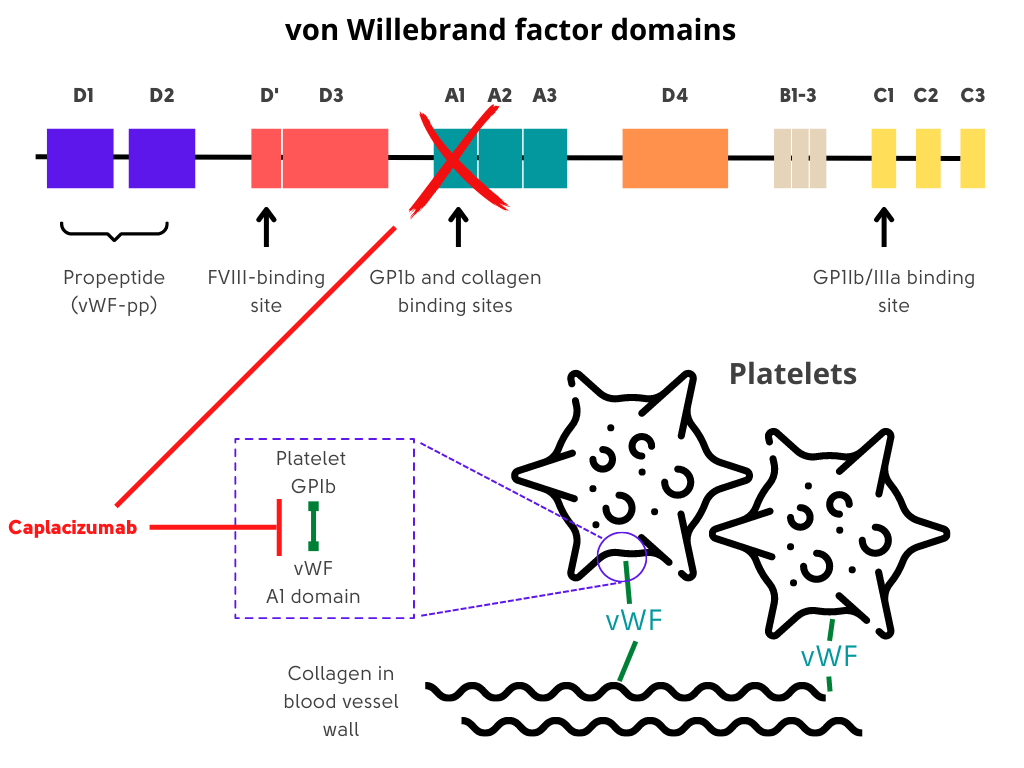
Suggested dosing:
- Day 1 – 11 mg bolus IV injection ≥ 15 minutes before plasma exchange, followed by 11 mg subcutaneous injection after end of plasma exchange
- Subsequent days of treatment during daily plasma exchange: 11 mg subcutaneous injection once daily followed by plasma exchange
- After plasma exchange treatment period: 11 mg subcutaneous injection once daily for 30 days following last daily plasma exchange; if after initial course of treatment, signs of underlying disease such as low ADAMTS13 activity level persists, consider extending caplacizumab treatment for maximum 28 days.
Notes:
- Caplacizumab does not correct underlying ADAMTS13 deficiency nor does it eliminate anti-ADAMTS13 autoantibodies.
- Discontinuation of treatment after normalization of platelet count but with persistently low ADAMTS13 activity (< 10% of normal) may result in exacerbation of disease.
- Immunosuppressive treatments such as rituximab and corticosteroids are still needed to manage underlying disease process.
TITAN study
- International, multicenter, phase 2, randomized, placebo-controlled study of caplacizumab.
- Seventy-five patients with acquired TTP were enrolled.
- Patients in both groups received the current standard of care of plasma exchange and immunosuppressive therapy.
- As compared with placebo, caplacizumab:
- Showed a short-term efficacy by reducing both:
- Time to platelet count normalization
- Early exacerbation rate
- Did not have an effect on the risk of relapse of TTP
- Associated with an increased tendency toward bleeding as compared with placebo, a potential outcome of blocking the interaction of von Willebrand factor with platelets.
- Showed a short-term efficacy by reducing both:
HERCULES study
- Phase 3 double-blind, randomized, parallel group, multicenter placebo-controlled trial designed to confirm the potential role of caplacizumab in the treatment of TTP.
- 145 patients with TTP randomized to receive caplacizumab (10-mg intravenous loading bolus, followed by 10 mg daily
- Subcutaneously) or placebo during plasma exchange and for 30 days thereafter.
- As compared with placebo, caplacizumab was associated with:
- Greater percentage of patients to achieve normalization of the platelet count
- Shorter median time to normalization of the platelet count
- Lower recurrence of TTP during the trial
- Less plasma exchange
- Shorter hospitalization than those who received placebo
- Lower incidence of a composite of
- TTP-related death
- Recurrence of TTP
- Thromboembolic event
- Higher rate of mucocutaneous bleeding
Guideline recommendations:
ISTH guidelines for treatment of thrombotic thrombocytopenic purpura:
- For patients with immune TTP experiencing an acute event (first event or relapse), the panel suggests using caplacizumab over not using caplacizumab. (A conditional recommendation in the context of moderate certainty evidence.)
- Benefit of caplacizumab is accrued if it is started in the early phase of an acute TTP event (i.e., at the time when a diagnosis is confirmed). Practically, when treating physicians are considering caplacizumab, they should consider administration of the drug even before the results of plasma ADAMTS13 activity become available.
- Caplacizumab should only be given under the guidance of an experienced clinician.
- Clinicians using caplacizumab and patients who receive it must understand its unique mechanism of action as caplacizumab does not correct the underlying ADAMTS13 deficiency nor does it eliminate autoantibodies against ADAMTS13, the primary cause of iTTP.
- Discontinuation of caplacizumab treatment after platelet count normalization but with persistently low ADAMTS13 activity(<10 U/dL) may result in disease exacerbation. Therefore, the immunosuppressive therapies such as rituximab and corticosteroids are still required to control the underlying disease process.
Prev
1 / 0 Next
