Labs
The following is the complete blood count (CBC) on the day you see the patient and a baseline CBC from 2 months earlier at the time of her valve repair:
| Day | WBC (109/L) | Hb (g/dL) | MCV (fL) | RDW-SD (fL) | PLT (109/L) |
|---|---|---|---|---|---|
| Day of admission | 11.8 | 7.3 | 101 | 67 | 264 |
| Preop (2 months ago) | 8.4 | 12.5 | 92 | 41 | 235 |
What’s what: WBC, white blood cell count; Hb, hemoglobin; MCV, mean cell volume; MCHC, mean cellular hemoglobin concentration; RDW-SD, red cell distribution width-standard deviation; platelets, PLT; Normal values: WBC 5-10 x 109/L, RBC 4-6 x 1012/L, Hb 12-16 g/dL, Hct 35-47%, MCV 80-100 fL, MCHC 32-36 g/dL, RDW-SD < 45 fL, platelets (PLT) 150-450 x 109/L
Reticulocyte count on day of admission:



The reticulocyte count is reported as 5.6%… 5.6% of what?
Reticulocyte count on day of admission:



The absolute reticulocyte count is reported as 0.15 m/ul or 150 x 109/L? The patient’s reticulocyte count is similar to which other blood count?
Correct. A normal platelet count is 150-450 x 109/L.
The patient, then, appears to have acute or subacute anemia (developing sometime over the previous 2 months) which is hyperproliferative based on an appropriate reticulocyte count and macrocytic probably owing to the reticulocytosis. Let’s consider the hemolysis labs:
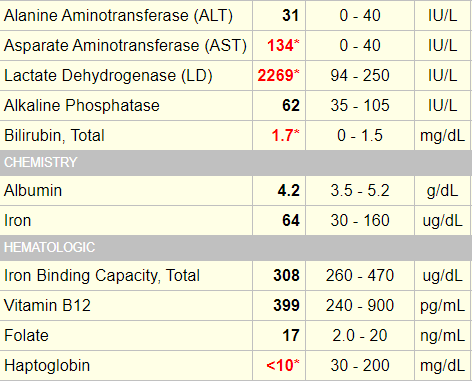

Classic for hemolysis, right? Elevated LDH, AST:ALT ratio and bilirubin; and low (in fact, undetectable) haptoglobin.
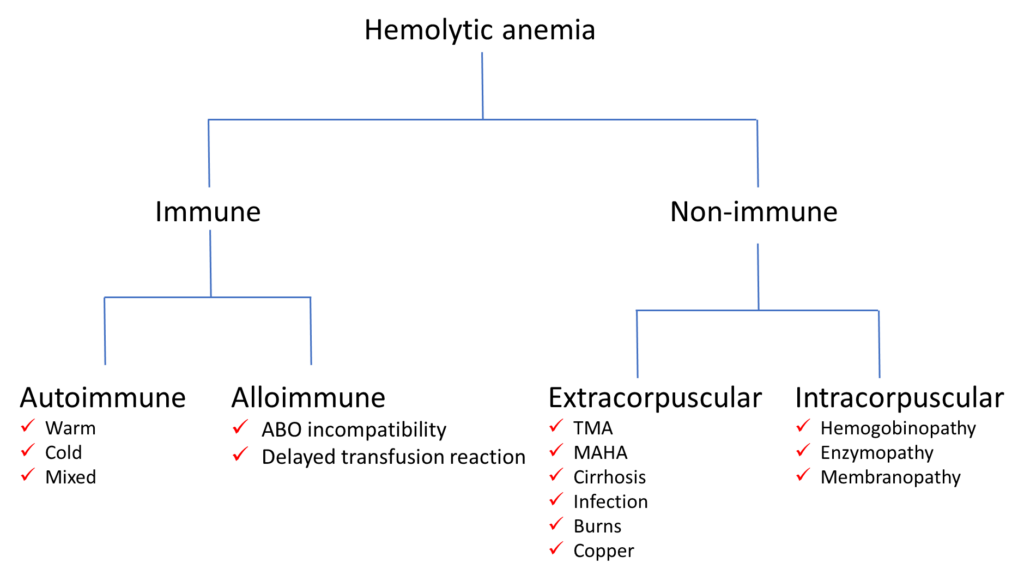
Hemolysis can be classified in several ways:
- Congenital vs. acquired
- Immune vs. non-immune
- Extracorpuscular vs. intracorpuscular
- Intravascular vs. extravascular
Intravascular hemolysis describes the lysis or rupture of red cells inside the circulation with leakage of intracellular contents into the bloodstream. Extravascular hemolysis refers to the ingestion of red cells by macrophages and the subsequent intracellular degradation of hemoglobin into its constituent parts.















Do these lab results distinguish between intravascular and extravascular hemolysis?
Click for AnswerLDH, AST, bilirubin and haptoglobin may be abnormal in both intravascular and extravascular hemolysis because:
- Many cases of hemolysis involve a component of both intravascular and extravascular hemolysis.
- In intravascular hemolysis, the haptoglobin-hemoglobin (Hb) complex is internalized by macrophages leading to Hb degradation and bilirubin production.
- In extravascular hemolysis, there may be “back leak” of red blood cell products (e.g., LDH, AST and Hb) into the circulation.
However, the presence of free hemoglobin in plasma and hemoglobin in the urine is more common and more pronounced in intravascular hemolysis.
For more details, click here.
Here are the results of the uranalysis:


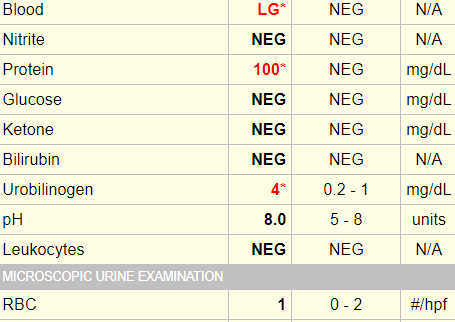

The presence of blood on dipstick with virtually no red cells on microscopy is diagnostic of pigment in the urine, either hemoglobin or myoglobin. Distinguishing between these two pigments requires mass spec analysis which is rarely available or performed. Diagnosis is based on the clinical context (conditions associated with hemolysis vs. rhabdomyolysis).
















There is a urine test that may be used to retrospectively detect presence of hemolysis. What is it?
Click for AnswerUrine hemosiderin
Hemoglobin that is filtered by the kidneys is reabsorbed in the proximal convoluted tubule (PCT). PCT cells process the hemoglobin much the same way as macrophages do, only they hold onto the iron (instead of releasing it to feed erythroid precursors) and store is as ferritin or hemosiderin. The latter is a complex of iron with phosphate and hydroxide forms that is formed when the capacity for storage of iron in ferritin is exceeded. All PCT cells undergo turnover. When they die, they slough off with the hemosiderin and are excreted into the urine, where they can be detected using a Prussian blue stain. Urine hemosiderin is usually seen three to four days after the onset of hemolytic conditions.
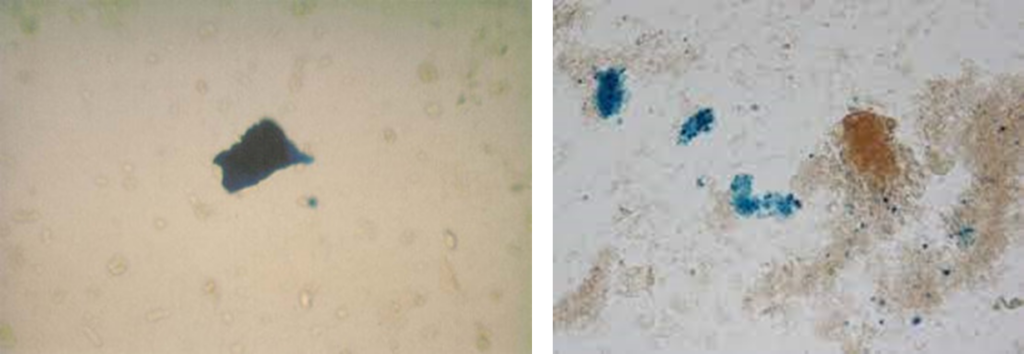

Urine hemosiderin was not performed in our patient.
I hope that you agree we have secured a diagnosis of hemolytic anemia.
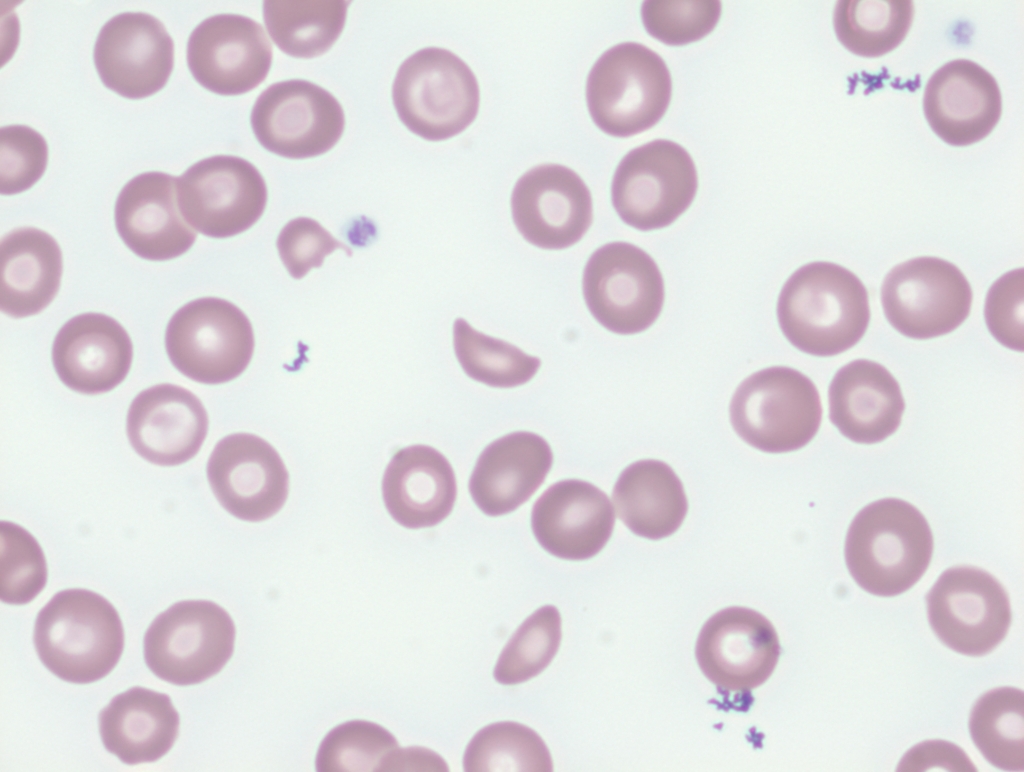
The following is similar to the peripheral blood smear from the patient:

Differential diagnosis of hemolytic anemia
Recall our simple classification of causes of hemolytic anemia from the previous section:

Now let’s reorganize the causes of hemolytic anemia in table format and consider the peripheral smear findings. Clostridial sepsis and babesiosis are included as infectious causes. Can you fill in the table (in your head) before looking at the descriptions on the next slide (we can assume that polychromatophilia applies to all causes)?
| Condition causing hemolytic anemia | Smear findings |
|---|---|
| Warm hemolytic anemia | |
| Cold hemolytic anemia | |
| Delayed transfusion reaction | |
| TMA | |
| MAHA | |
| Cirrhosis | |
| Infection – Clostridial sepsis | |
| Infection – Babesiosis | |
| Burns | |
| Thalassemia | |
| Sickle cell disease | |
| PK deficiency | |
| G6PD deficiency | |
| Hereditary spherocytosis | |
| Hereditary elliptocytosis | |
| PNH |
Now let’s reorganize the causes of hemolytic anemia in table format and consider the peripheral smear findings. Clostridial sepsis and babesiosis are included as infectious causes. Can you fill in the table (in your head) before looking at the descriptions on the next slide (we can assume that polychromatophilia applies to all causes)?
| Condition causing hemolytic anemia | Smear findings* |
|---|---|
| Warm hemolytic anemia | Spherocytes |
| Cold hemolytic anemia | Red cell agglutination |
| Delayed transfusion reaction | Spherocytes |
| TMA | Schistocytes, decreased platelets |
| MAHA | Schistocytes |
| Cirrhosis | Macrocytes, target cells, acanthocytes (spur cells) |
| Infection – Clostridial sepsis | Microspherocytes |
| Infection – Babesiosis | Intracellular parasites |
| Burns | Elliptocytes, microspherocytes |
| Thalassemia (minor) | Microcytes |
| Sickle cell disease | Sickle cells, nRBC, HJ bodies |
| PK deficiency | Acanthocyte-like cells |
| G6PD deficiency | Bite cells |
| Hereditary spherocytosis | Spherocytes |
| Hereditary elliptocytosis | Elliptocytes |
| PNH | May appear normal |
Our patient has schistocytes on her peripheral smear. According to the table above, that helps to narrow the likely diagnosis to TMA or MAHA.
Concerning thrombotic microangiopathy (TMA) vs. microangiopathic hemolytic anemia (MAHA), which of the following statements is true (only one answer applies)?
Thrombotic microangiopathy (TMA) is characterized by:
- Microangiopathic hemolytic anemia (MAHA)
- Thrombocytopenia
- Organ injury
Microangiopathic hemolytic anemia (MAHA) is characterized by red blood cell destruction within the microvasculature, and may occur in:
- TMA
- Extracorpuscular hemolysis due to mechanical trauma:
- Foot-strike anemia
- Percussion hemolysis
- Valve hemolysis (if one expands the definition of MAHA to include not just microvessels, but also a paravalvular leak.)
The previous slide mentioned 2 interesting, but rare causes of microangiopathic hemolytic anemia (MAHA):
Foot-strike anemia (also called runners hemoglobinuria):
- The first case was described in 1881 in a German soldier who demonstrated severe hemolytic anaemia reported the passage of dark urine after extended periods of marching on the field. Hence, it was initially called march hemoglobinuria.


- Since then, foot-strike hemolysis has been demonstrated to be the greatest contributor to anemia in athletes. The risk of developing hemolysis in runners depends on individual running style, type of foot wear and the running surface.


Percussion hemolysis
Hemolysis and hemoglobinuria have been reported in passionate hand percussion drum players, for example those playing following African drums. Percussion hemoglobinuria is very similar in pathogenesis to march hemoglobinuria, except that it occurs in drum players and the intravascular hemolysis occurs in the palms instead of soles.




In cases of acute hemolysis, ruling out thrombotic microangiopathy (TMA) is a priority because a diagnosis of thrombotic thrombocytopenia purpura (TTP) has a mortality rate of about 90% if left untreated. Even patients with a recent valve replacement may develop TTP.
These are the results of a transesophageal echocardiogram as they related to the mitral valve:


The diagnosis, then, is most certainly valve hemolysis.
Before moving on to treatment, we want to look at the renal function.
The patient’s renal function was normal.
Now let’s move on treatment considerations.
