What is evolutionary medicine, and why should doctors be interested?
Evolutionary medicine is not a specialty like hematology or a field like genetics. Instead, it consists of all the diverse areas of medical research and practice where evolutionary insights bring added value by connecting ideas, inspiring insights, or improving clinical outcomes. Here I present recently published examples of all three benefits of evolutionary thinking and provide suggestions for further reading.
Before doing that, let me introduce three major concepts that appear repeatedly.
First, organisms – including patients – are designed by evolutionary processes that result in pervasive tradeoffs. A tradeoff occurs when a change in one trait that improves evolutionary fitness is connected to changes in other traits that reduce evolutionary fitness. Tradeoffs evolve because evolution will accept any cost that is connected to an even greater benefit; large benefits trade off with large costs. In evolution reproduction is the greatest benefit, and the classical tradeoff is between reproductive performance early in life and survival later in life. The large benefit is babies that transmit genes to future generations; the impressive evolutionary costs are the inevitability of death for all and the risks of morbidity and mortality to the mother. Another benefit is survival, which is directly influenced by health and disease, for while evolution is focused on reproduction, one must survive to reproduce, and all traits associated with survival are also involved in tradeoffs. Thus, tradeoffs are pervasive, and we can expect that treatments that improve one aspect of health will often be associated with costs elsewhere, often in some structure or function that has not yet been identified.
Second, we did not evolve in the modern environment and therefore are not (yet) well adapted to it: postindustrial populations are experiencing mismatch between their inherited genes and their novel environments. This is another way to say that natural selection needs time to adapt a population to new circumstances, especially if those circumstances keep changing. Populations experience mismatch as impaired health and reduced survival.
Third, the number of times that an evolutionary event – a mutation, a birth, a death, an environmental challenge – occurs is very important, both in producing adaptations and in creating risks. Organs that are clearly adaptive and adapted – like the vertebrate eye, heart, brain, and kidney – have been finely polished in their function by billions of mutations over hundreds of millions of years, mutations that have explored many ways to improve or impair organ function.
The degree to which an adaptation is precise depends directly on how many selective events have occurred that affected it in the past – how many mutations, births, and deaths. Think of a sculptor carving a figure out of a block of marble (Fig. 1). If she could only deliver 10 blows of the chisel, she would get a very rough piece of stone. But if she makes thousands of blows of the chisel, then rubs with millions of grains of sandpaper, she can produce a masterpiece. The precision of an adaptation depends not only on the difference that a gene substitution makes to lifetime reproductive success; it also depends on how many times mutations shaping the trait have been fixed. Those that improved function increased in frequency, spreading through populations, and are largely the ones we have each inherited. Some of them are now very precise, and that is why most novel mutations are now detrimental: most of the improvements have already been found. Note that adaptations are not produced by processes that happen infrequently, such as species extinctions.

As for creating risks, our bodies have been produced by roughly 40 trillion cell divisions, and each time a cell has divided, there has been an opportunity for a somatic mutation at some site. The average mutation rate per nucleotide in humans is about one in a billion per cell division, and there are about 3.3 billion nucleotides in our genome. Thus, at each cell division, about 3 nucleotides mutate, and during growth from a single celled zygote to a fully grown adult, every individual experiences about 120 trillion somatic mutations. The probability of winning a big payoff in the National Lottery in the UK is about 1 in 100 million. Cancer is given enough tickets to win the lottery many times over in each human. We can thank evolution for having produced defense mechanisms that make multicellularity possible. It has been working on the problem for about 2 billion years. Mutations that severely compromise function cause the cells that carry them to commit suicide; the cells that survive – all of which now have some somatic mutations – are at risk of slipping out of control and causing cancer. Every multicellular organism has evolved quality control mechanisms that survey cell function and are quite good, but not perfect, at greatly reducing the risk of cancer.
Connecting ideas
In 2019, Wang, Luan, and Medzhitov published a remarkable paper in Science, in which they used insights from life history evolution1 to connect the endocrine and immune systems and thereby to explain the mechanisms that mediate the metabolic connections among growth, reproduction, and maintenance. Maintenance programs are engaged when environmental conditions are unfavorable and include dormancy and immune defense. It is the connections among those programs that create the critical tradeoffs shaping the evolution of age and size at maturity, size and number of offspring, and lifespan. While well worth reading in detail, the take-home messages of that paper can be summarized in the three figures reproduced here.
The first communicates the idea that our endocrine system regulates the balance between catabolism and anabolism, with anabolism engaged in growth, reproduction and defense under favorable conditions and catabolism mediating dormancy under unfavorable conditions.
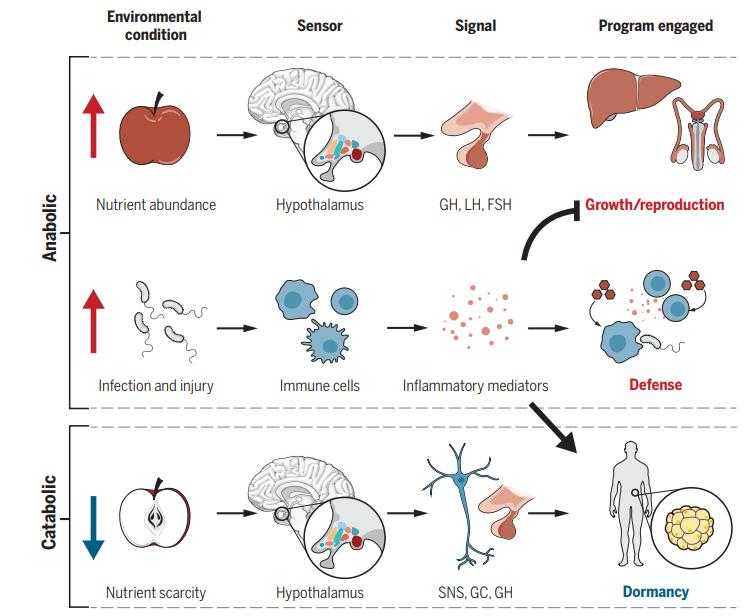
Figure 2. A life history perspective of metabolic programs. In favorable environmental conditions, growth and reproduction programs are engaged, which rely on anabolic metabolism. Under unfavorable environmental conditions, maintenance programs are engaged. There are two types of maintenance programs: dormancy and defense. Dormancy is induced by nutrient scarcity and relies on energy preserving catabolic metabolism, whereas defense is induced by infections (and other hostile factors) and requires the support of anabolic metabolism. GH, growth hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; SNS, sympathetic nervous system; GC, glucocorticoid. From Wang, Luan, and Medzhitov.
The second shows how the hypothalamus serves as a central controller of organismal life history programs. By sensing changes in the environment and then engaging other organs such as the liver, reproductive organs and adrenal gland, the hypothalamus coordinates growth, reproduction, and maintenance, thus managing the energetic tradeoffs that occur within every organism as it deals with environmental challenges.

Figure 3. Hypothalamus as a central coordinator of organismal life history programs. The growth, reproduction, and maintenance arms of life history theory correspond on the organismal level to the growth hormone – insulin-like growth factor (GH-IGF), hypothalamic – pituitary – gonadal (HPG), and hypothalamic – pituitary – adrenal (HPA) axes, respectively. These axes all initiate at the level of the hypothalamus, and can be engaged or disengaged depending on inputs reporting on the quality of the environment. The GH-IGF axis regulates hepatic secretion of IGF-1, which is known to be essential to growth. The HPG axis controls the gonadal secretion of sex hormones, which are necessary for reproductive maturation and function. The HPA axis governs adrenal secretion of glucocorticoids, which are a common component of responses to environmental stress. From Wang, Luan, and Medzhitov.
The third uses this conceptual framework to show how the inflammatory response to infection suppresses growth and reproduction and activates a maintenance program that includes both defense (resistance that relies on anabolism and promotes pathogen clearance) and dormancy (tolerance that relies on catabolic programs and promotes tissue protection).
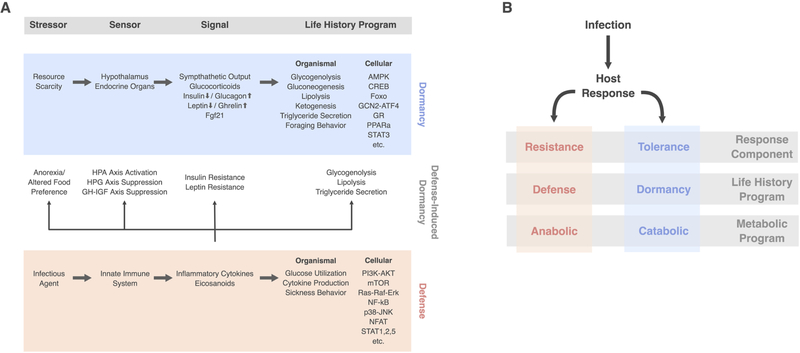
Figure 4. Dormancy and defense are distinct programs of maintenance. Favorable environments promote investment into growth and reproduction. Unfavorable environments are of two types – resource scarcity and presence of insult (pathogen, toxin, etc.) – and both lead to divestment in growth and reproduction. A. Resource scarcity induces dormancy states, which are characterized by divestment in non-essential functions, energy conservation, and reliance on catabolic metabolism. These programs are generally tissue- and cyto-protective. B. Presence of insults induces defense states, which are characterized by energy consumption and anabolic metabolism. Components of the system that are not required for defense engage dormancy both for protection and to divert resources to the high energy consuming defense arm. From Wang, Luan, and Medzhitov.
Why is this study important? It creatively combines life history evolution with endocrinology and immunobiology to present a detailed picture of how organisms are designed at that level for reproductive success. It significantly advances the newer field of evolutionary physiology, complementing and extending the older field of comparative physiology. In so doing, it appeals to the interests and abilities of a broad community of biologists helping recruit them to life history evolution and evolutionary medicine by exposing them to neat questions and intriguing problems. It thus helps to unify parts of biology that had been fragmented, sending them together into a future shaped by big questions. Perhaps even more important is the integrated overview that it creates of the organism – the patient – inspired by insights from life history evolution. It places any developmental or physiological process into a larger framework, a framework shaped by tradeoffs and designed by evolution to promote reproductive success.
Inspiring insights
In an equally remarkable paper published in Nature Ecology and Evolution in 2019, Kshitiz et al. used a combination of powerful techniques to test the idea that species with more invasive placentas are at greater risk of metastatic cancer.2
Here’s some background. Humans and other primates belong to a lineage that has highly invasive placentas. That means that when the fertilized zygote has moved down the Fallopian tube and divides to form a multicellular trophoblast, it starts the process of embedding into the endometrium and forming the placenta by invading maternal tissue. To do so, cells from the trophoblast must detach and move through the endometrium. Some of them embed into the walls of the maternal arterioles that are growing out into the developing placenta; others form elements of the endocrine tissue that will secrete hormones into the maternal bloodstream, creating the capacity to influence maternal blood pressure and blood glucose. The ability of a cell to migrate through foreign tissue and establish itself in a new environment where it can absorb nutrients and multiply are precisely the capacities needed by a metastatic cancer cell. Because our developmental genetics is so organized that every cell in the body that contains a nucleus has all the genetic information needed to support all types of cells in the body, every nucleated cell in our body contains all the information needed to support metastasis.
Note the tradeoff: performance very early in life is linked to survival risk much later in life.
To test that idea, Kshitiz et al. exploited a powerful comparison handed to them by evolution: cows, horses, and other ungulates, which belong to a lineage that does not have invasive placentation, develop solid tumors but are at very low risk of metastasis. They grew cow and human stromal cells (fibroblasts) in vitro and then monitored the growth of cow and human trophoblast cells on both of those two-dimensional lawns. They also grew melanoma cells on both cow and human substrates, and they sampled gene expression to see if which genes were involved in both invasion and metastasis. They then targeted those genes with small interfering RNAs (siRNAs) to test their causal role in mediating invasion. They demonstrated that cultures of human cells are more permissive to invasion by trophoblasts and melanoma cells than are cultures of cow cells, and they identified the genes involved, which are now targets for therapies. They then extended their analysis by culturing cells from rabbits, rats, guinea pigs, cats, horses, and sheep, yielding comparisons in a phylogenetic tree with eight species. That phylogenetic framework allowed them to conclude that what had happened in evolution was not that human embryos had become more invasive; instead, human mothers had become more permissive, a trait probably shared with all primates. Metastatic potential thus appears to be determined, at least in part, by the permissiveness of stromal cells.
Thus, species differences in malignancy rates appear to be caused in part by differences in the invasibility of the endometrial stroma. Critical events very early in reproduction are connected to and in part responsible for the risk of metastatic cancer much later in life.
This figure summarizes their experimental platform and illustrates the difference in rates of invasion of human and bovine endometrial stroma:

Figure 5. An experimental platform to test the hypothesis of Evolved Levels of Invasibility (ELI). (A) Schematic describing the ELI hypothesis. Placentation in humans is hemochorial, wherein the placental trophoblasts invade into the maternal stroma reaching the blood supply. In contrast, in cows and other boroeutherians, placentation has recently evolved to be epitheliochorial, where the trophoblast epithelium attaches to the endometrial epithelium, but does not invade the maternal interstitium. The ELI paradigm states that bovine stroma has evolved to resist invasion compared to human stroma, and therefore secondarily limits cancer metastasis. (B) Schematic showing a cell patterning nanotextured platform to quantitatively and sensitively measure collective invasion into stroma; Stromal cells and invasive cells are patterned by a PDMS stencil into juxtaposed monolayers heterotypically interacting with each other, and imaged using live cell microscopy to observe collective cell invasion into the stroma. (C) Time course images showing invasion of 1205Lu malignant melanoma cells (red) into BJ5ta human skin fibroblasts (unlabeled) for 18 hours on a flat substrate vs a nanotextured substrate; Quantification of the extent of invasion per unit length of heterotypic intercellular interaction shown in (D). (E) Quantification of the extent of invasion of non-malignant WM35 and malignant 1205Lu melanoma into a monolayer of BJ5ta human skin fibroblasts on flat and nanotextured substrata. (F) Time course images showing invasion of human choriocarcinoma derived trophoblasts, J3 (red) into human endometrial stromal fibroblasts (unlabeled); and bovine trophoblasts, F3 (red) into bovine stromal fibroblasts (unlabeled) for 48 hours; Quantification of the extent of invasion of trophoblasts into the respective stromal monolayer shown in (G). (H) Time course dynamic analysis showing cumulative invasion of J3 and F3 into respective species-specific endometrial stromal monolayers. In D, E, and G, n = 4 independent biological replicates; Statistical comparisons made using Student’s t-tests **: p < 0.01, ***: p < 0.001; Error bars denote standard error of the mean (s.e.m.). From Kshitiz et al.
Note that the mechanisms used in metastasis are shared by processes that predate the evolution of placentation. The mechanisms that cells use to metastasize, gastrulate, and move out of blood vessels – all present in marsupials, which lack placentas – are shared with trophoblast invasion. Invading cancer cells thus use mechanisms that evolved earlier than placental invasion, which cannot be responsible for the origin of malignant cancer. The invasiveness of the placenta continued to evolve after its origin. Placental invasion reverted to a non-invasive phenotype in several lineages of placental mammals, including the ungulates. Meanwhile, it evolved even higher invasiveness in the great apes, including humans.
Why is this study important? It creatively combines the comparative method with powerful technologies to suggest potential therapies. It reveals a tradeoff between performance extremely early in life with cancer risk much later in life. We have no reason to think that this is the only such connection. It supports – at least for this type of question – Günter Wagner’s claim: “Nothing in evolution makes sense except in the light of mechanism“.3
Improving clinical outcomes
The rapid evolution of antibiotic resistance poses a major global threat to our ability to treat bacterial infections. Multiply drug resistant (MDR) strains now circulate in most hospitals and many communities, and it only takes a year or two for resistance to new antibiotics to evolve and spread across the globe. This problem has focused interest on the development of evolution-proof antimicrobial therapies. One possibility is phage therapy, the use of viruses that attack bacteria but not eukaryotic cells to control bacterial infections. Phage therapy has a long history, mostly in Eastern Europe, but it did not become a priority in the West until bacteria evolved resistance to most antibiotics. Of course, if bacteria can evolve resistance to antibiotics, they can also evolve resistance to phage, and so it takes an additional feature to make phage therapy evolution-proof. That feature was discovered and implemented by Chan, Turner and their colleagues (Chan et al. 2018, Kortright et al. 2019).4 They had a very clever insight that exploits the concept of tradeoffs to trap bacteria in an unavoidable dilemma: they use a phage that attacks part of the mechanism that the bacterium uses to resist antibiotics. They let the bacterium evolve resistance to the phage, which causes it to lose its resistance to the antibiotic, and then apply the antibiotic, which clears the infection. The insight can also be used to guide the evolution of bacterial virulence by using phage to attack the virulence mechanism:

Figure 6. Renewed Approach to Phage Therapy: Phage Selection against Virulence or Antibiotic Resistance. Certain lytic phage may be more effective in phage therapy, because they kill target bacteria while simultaneously imposing strong selection against bacterial virulence or antibiotic resistance when bacteria mutate to avoid phage attack. Phage that use antibiotic efflux pumps as receptors (red) can select for phage-resistant bacterial mutants with impaired efflux pumps; these phage-resistant bacterial mutants are more sensitive to antibiotics. Phage that bind to structural virulence factors such as a capsular antigen (purple) can select for phage-resistant bacterial mutants that lack the capsule; these non-capsulated phage-resistant mutants are less virulent because they are more easily engulfed by phagocytic cells. From Kortright et al. 2019.
The first patient on whom this approach was tried was an elderly physician who had a prosthetic aortic vascular graft that had been infected by MDR Pseudomonas aeruginosa and only expected to live for a few more months. They screened a library of phage to locate one, OMKO1, that attacked the bacterial efflux pump. A single application of phage, followed by treatment with ceftazidime, resolved the infection with no sign of recurrence (Chan et al. 2018).5 This is what the aortic graft looked like prior to treatment:

The approach has been used to treat multiple patients suffering from cystic fibrosis whose lungs are infected by MDR Pseudomonas aeruginosa, with very promising results (Chan et al. 2021, Ng et al. 2021).6
This summary of case studies and clinical trials was published in 2019 (Kortright et al. 2019):7
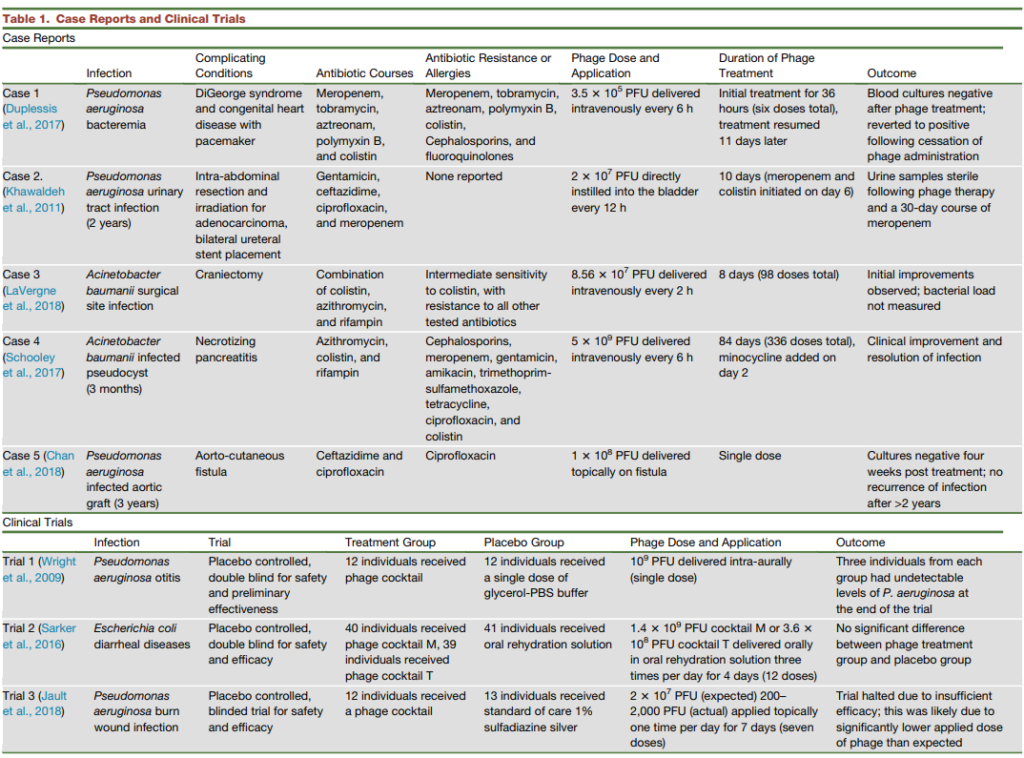
The following are details on one unpublished case:
An immunosuppressed elderly patient with a history of brain aneurysms had vertebral osteomyelitis complicated by hardware that could not be removed from posterior spinal fusion. Her wound dehisced exposing hardware with deep tissue cultures growing Pseudomonas aeruginosa. She went back for incision and drainage of the dehisced spinal hardware infection followed by treatment with 2 antibiotics. She could not be treated with fluoroquinolone because of the risk of aneurysm rupture. Without phage therapy she would have had to stay on IV antibiotics until resistance evolved, treatment failed, and she died. On IV antibiotics, she suffered worsening fatigue, ongoing wound drainage, dehydration characterized by orthostatic hypotension, new transfusion dependent anemia of chronic disease, poor appetite, and repeated flares of rheumatoid arthritis. She was given phage therapy via IV, instillation into her bladder, and into the spinal hardware drain. It completely cured her bacterial infection and greatly improved the quality of her remaining life. (Ben Chan, pers. comm.)
Why are these findings important? Phage therapy that targets bacterial mechanisms of resistance and virulence looks very promising and stands a good chance of becoming a standard tool for dealing with certain classes of bacterial infections. It addresses a problem caused by evolution, uses an insight into tradeoffs inspired by evolutionary biology, and has the potential to become an evolution-proof antimicrobial therapy. In helping to save lives and limbs that would otherwise have been lost, it helps to preserve surgery as we knew it in the golden age of antibiotics. This is a compelling reason to adopt evolutionary thinking in appropriate parts of medical research and practice.
Further reading
Two papers8 and a textbook9 provide reviews of recent progress in evolutionary medicine. Corbett and colleagues (Corbett et al. 2018) present a broad overview of the origins and consequences of mismatch on postindustrial populations.10 Byars and colleagues (Byars et al. 2017) dissect another aspect of the tradeoff between reproduction and survival, showing many of the alleles that increase the risk of coronary artery disease later in life also improve some aspect of reproduction or survival early in life.11
About the author
Stephen Stearns received his PhD in Zoology from the University of British Columbia, followed by a Miller Fellow at the University of California Berkeley, and academic appointment as an Assistant Professor of Biology at Reed College in Portland, OR. From there, he became Professor of Zoology at the University of Basel, Switzerland, where he spent 17 years. He was recruited by Yale in 2000 and retired in July 2021. In addition to writing a number of books on evolution and evolutionary medicine, Dr. Stearns helped to found and served as president of the European Society for Evolutionary Biology, the Tropical Biology Association, and the International Society for Evolution, Medicine, and Public Health. He was founding editor of the Journal of Evolutionary Biology and Evolution, Medicine, and Public Health. Click here to learn more.
